Question
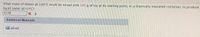
Transcribed Image Text:What mass of steam at 100°C must be mixed with 185 g of ice at its melting point, in a thermally insulated container, to produce
liquid water at 60°C?
|43.08
X 9
Additional Materials
eBook
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- A container which is air tight has a volume of * 2 * 500L internal pressure of 1.0 atmosphere, internal temperature of 15 °C is washed off ythe deck of a ship located in the Philippine Trench, and sinks to a depth where the pressure is 175 atmosphere and the temperature is 3 °C. What would be the volume in m³ of the gas inside be when the container breaks under the pressure at this depth?arrow_forwardA cube of ice is taken from the freezer at -8.5°C and placed in a 100 gram aluminum calorimeter filled with 300 grams of water at a room temperature of 20°C. The final situation is observed to be all water at 17°C. What was the mass of the ice cube? Given the following values: Cice =2100 J/kg/°C, Cwater = 4186 J/kg/°C, Caluminum 920 J/kg/°C, and for water Le 3.33×105 J/kg and Lv = 22.6×105 J/kg. 1arrow_forwardWhat mass of water at 28.0°C must be allowed to come to thermal equilibrium with a 1.70-kg cube of aluminum initially at 150°C to lower the temperature of the aluminum to 62.0°C? Assume any water turned to steam subsequently recondenses. kgarrow_forward
- A styrofoam cooler (k = 0.030 W/(m-°C) has outside dimensions of 0.170 m x 0.250 m x 0.260 m, and an average thickness of 2.0 cm. How long will it take for 1.10 kg of ice at -5.0°C to melt (to water at 0°C) in the cooler if the outside temperature is 34.0°C? Assume that ice temperature increases linearly from -5.0°C to 0°C. Neglect any temperature change of the air in the cooler. Data: Specific heat of ice is 2050 J/(kg-K). Laten heat of fusion of water is 3.34×105 J/kg.arrow_forwardWhat mass of water at 28.0°C must be allowed to come to thermal equilibrium with a 1.75-kg cube of aluminum initially at 150°C to lower the temperature of the aluminum to 66.0°C? Assume any water turned to steam subsequently recondensesarrow_forwardSuppose 6.6 kg of chloroform at 24.4°C is poured into 6.6 kg of propylene glycol at 37°C. Calculate the final equilibrium temperature, neglecting any energy processes associated with mixing, given Cprop = 2500 J/(kg-K) and Cchloroform = 1050 J/(kg-K). T = °Carrow_forward
- What mass of water at 20.0°C must be allowed to come to thermal equilibrium with a 1.95-kg cube of aluminum initially at 150°C to lower the temperature of the aluminum to 60.0°C? Assume any water turned to steam subsequently recondenses. kgarrow_forwardIce has formed on a shallow pond, and a steady state has been reached, with the air above the ice at -7.6°C and the bottom of the pond at 3.7°C. If the total depth of ice + water is 2.3 m, how thick is the ice? (Assume that the thermal conductivities of ice and water are 0.40 and 0.12 cal/m-Cº-s, respectively.) Number Unitsarrow_forwardMost automobiles have a coolant reservoir to catch radiator fluid that may overflow when the engine is hot. A radiator is made of copper and is filled to its 11.5-L capacity when at 11.0°C. What volume of radiator fluid will overflow when the radiator and fluid reach their 95.0°C operating temperature, given the fluid's volume coefficient of expansion is 400 ✕ 10^−6/°C and that of copper is 51 ✕ 10^−6/°C. Note that most car radiators have operating temperatures of greater than 95.0°C.arrow_forward
arrow_back_ios
arrow_forward_ios