
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Ti is 25C
I do not know what Tf is because I put 40 and it was wrong
Can you use this graph to help find Tf and use that to answer the equation

Transcribed Image Text:If 7.5 g of KBr were dissolved in 100. g of water, how much heat (kJ) was involved? (2
Significant Figures)
kJ
J
(q = msolution*C*AT where the heat capacity of the solution is 4.18
g°C
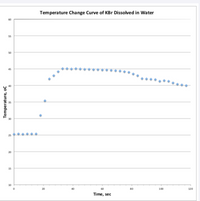
Transcribed Image Text:Temperature Change Curve of KBr Dissolved in Water
45
25
20
15
10
20
40
60
80
100
120
Time, sec
Temperature, oC
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Balance the equationsarrow_forwardAn unknown solution shows the following observations. State whether each is a positive or negative test result. (Insert Image) What are the ions present in the solution?arrow_forwardBalance the following reaction in basic solution Cr20,2 (aq) + SO2(aq) → Cr³*(aq) + SO3(aq) Coefficients: Note: enter 1 for compounds that show up once in the reaction, enter 0 for compounds that do not appear in the balanced reaction. Cr20,2- SO2 Cr3+ SO3(aq) OH H* H2Oarrow_forward
- If 4.88 moles of F_{2} are reacted with 10.04 moles of Ti how many moles of Ti * F_{4} will result? Ti+2F 2 TiF 4arrow_forward26arrow_forwardBecause the limiting reactant in this case will be SCN- (from KSCN), the moles of SCN- in solution will determine the moles (and concentration) of FeSCN²+ in each standard solution. Concentrations and volumes are provided in the experiment procedure in Table 4.1. Use the concentration of KSCN, volume of KSCN and total final volume of each solution (each standard solution will be 20.00 mL) to calculate the FeSCN²+ concentration in each of the four standard solutions. 1 Fe³+ (aq) iron (III) 1. FeSCN²+ concentration in Standard Solution 1 = 234 + 4 SCN- (aq) thiocyanate TABLE 4.1. Volumes for the Part A standard solutions for the Beer's Law Plot. Solution Volume of FeSCN²+ (aq) thiocyanoiron (III) 5.00 5.00 5.00 5.00 Volume of 0.200 M Fe(NO3)3 (ml) 0.00200 M KSCN (mL) 2.00 1.50 1.00 0.50 Reaction 1 Volume of Deionized Water (mL) 13.00 13.50 14.00 14.50 Continued on next page...arrow_forward
- Balance the reaction between Fe and HNO3 to form Fe2+ and NO in acidic solution. When you have balanced the equation using the smallest integers possible, enter the coefficients of the species shown. Fe + HNO3 Fe2+ + NO (reactant, (Enter 0 for neither.) Water appears in the balanced equation as a product, neither) with a coefficient of How many electrons are transferred in this reaction?arrow_forwardI need the equation for Ni2+(aq) plus sodium hydroxide and dimethylglyoximearrow_forwardWe don't see the answer written with a photo or pen, give the answer using the toolarrow_forward
- A researcher wants to make a buffer using potassium dihydrogen phosphate. What mass of potassium dihydrogen phosphate in grams would the researcher need to make 1.0 liter of buffer with a concentration of 20. mM solution?arrow_forwardPlz do Asap...!arrow_forwardFor each reaction in the table below, write the chemical formulae of any reactants that will be oxidized in the second column of the table. Write the chemical formulae of any reactants that will be reduced in the third column. reaction JC1, () +2A1() SC(s) +S() 1₂(0)+ Ma(0) ZAICI, () Cas() Mal₂ (0) reactants oxidized 0 0 0 reactants reduced 0 0 0 Xarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY