
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
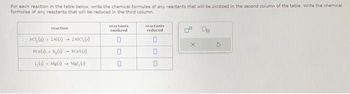
Transcribed Image Text:For each reaction in the table below, write the chemical formulae of any reactants that will be oxidized in the second column of the table. Write the chemical
formulae of any reactants that will be reduced in the third column.
reaction
JC1, () +2A1()
SC(s) +S()
1₂(0)+ Ma(0)
ZAICI, ()
Cas()
Mal₂ (0)
reactants
oxidized
0
0
0
reactants
reduced
0
0
0
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following metals, when exposed to iron (III) chloride, will be oxidized (i.e. initiate a redox reaction)? Group of answer choices Aluminum (Al) Copper (Cu) Tin (Sn) Gold (Au) Lead (Pb)arrow_forwardWhy is cellular respiration an example of a redox reaction? O 81°F Mostly cloudy Glucose is broken down to produce energy. ATP is produced. It is a single transfer of electrons in which compounds are oxidized and reduced. O There is a long series of electron transfers in which compounds are oxidized and reduced. F 3 O Search 78866 $ 4 5 A F8 & 7 * 8 ( a ) 0 4arrow_forwardRecognizing reduction and oxidatioh For each chemical reaction listed in the table below, decide whether the highlighted atom is being oxidized or reduced. highlighted atom is being... neither oxidized nor reduced reaction oxidized reduced C(s)+O,(9) → CO,(9) 2 Cro (a9)+2H,0 (aq)→ Cr,0, (a9)+3 H,O() 4 KI(aq)+2 CuCl,(aq)2 CuI(s)+I,(aq)+4 KCl(aq) 2H,S(aq)+O,(9) →2 S(s)+2 H,O(1)arrow_forward
- What element is being reduced in the following reaction: C6H12O6(s) + 4 KClO3(l) → 4 KCl(s) + 6 CO2(g) + 6 H2O(g) When I do this problem I keep getting Cl being reduced which feels wrong. Can you please help?arrow_forwardtab In each of the following reactions, identify the reactant that is oxidized and the reactant that is reduced: aps lock esc EEB F1 Q A 5,394 7927 @ 2 N F2 W JUL S ㅁㅁ X F3 E D $ 4 C F4 Part A Ba(s) + Cl₂(g) → BaCl₂ (s) TEL CHOCOL O Cl₂ gains electrons and is oxidized. Ba loses electrons and is reduced. O Cl₂ loses electrons and is oxidized. Ba gains electrons and is reduced. O Ba gains electrons and is oxidized. Cl₂ loses electrons and is reduced. O Ba loses electrons and is oxidized. Cl₂ gains electrons and is reduced. Submit Part B R Br2(g) + 2KI(aq) → 2KBr(aq) + I2 (s) O Br₂ loses electrons and is oxidized. I O Br2 gains electrons and is oxidized. I (in KI) gains electrons and is reduced. (in KI) loses electrons and is reduced. O I (in KI) gains electrons and is oxidized. Br₂ loses electrons and is reduced. OI (in KI) loses electrons and is oxidized. Br2 gains electrons and is reduced. Request Answer Submit Request Answer F 45 % F5 T V MacBook Air 6 G F6 Y B & 7 útv H F7 2 U N ➤11…arrow_forwardIn a redox reaction, electrons are transferred from the substance being oxidized to the reducing agent. O from the oxidizing agent to the reducing agent. from the substance being reduced to the oxidizing agent. from the substance being oxidized to the substance being reduced. O from the substance being reduced to the substance being oxidized DOO00arrow_forward
- For each reaction, write the chemical formulae of the oxidized reactants in the space provided. Write the chemical formulae of the reduced reactants in the space provided. reactants Mg oxidized: CuCl, (ag) + Mg(s) - MgCl, (ag) + Cu(s) reactants CuCl, reduced: reactants oxidized: 2AGNO, (ag) + Mg(s) 2Ag(s) + Mg(NO,), (aq) → reactants reduced: reactants oxidized: Feso, (ag) + Mg(s) → Fe(s) + MgSO, (aq) reactants reduced:arrow_forwardFor each reaction, write the chemical formulae of the oxidized reactants in the space provided. Write the chemica space provided. reactants oxidized: Zn (s) + 2A£NO, (aq) → - Zn (NO,), (aq) + 2Ag(s) reactants reduced: reactants oxidized: Fel, (aq) + Mg(s) - MgI, (aq) + Fe(s) reactants reduced: reactants oxidized: 2Fe(s) + 3CUCI, (aq) → 2FEC1, (aq) + 3Cu(s) reactants reduced:arrow_forwardHow many total electrons are transferred during the reaction of the oxidation of oxidation according to the following reaction? C(s) + O2(g) --> CO2(s)arrow_forward
- 6. 2 N2O → 2 N2+O2 redox reaction (yes/no) species oxidized oxidizing agent Oie species reduced reducing agentarrow_forwardIn the following oxidation-reduction reaction, what is being oxidized? C4H8 + 6 02 → 4 CO2 + 4 H20 O CO2 O C4H8 O H2Oarrow_forwardWhich substance is oxidized in each reaction? a) 2Zn(s) + O2(g) = 2ZnO(g) b) CH4(g) + 2 O2(g) = CO2(g) + H2O(g) c) Sr(s) + F2(g) = SrF2(s)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY