If 1.2 mol of oxygen gas is confined in a 10-L bottle under a pressure of 3.0 atm , what is the average translational kinetic energy of an oxygen molecule? Express your answer using two significant figures. Hν ΑΣφ к- J Submit Request Answer
If 1.2 mol of oxygen gas is confined in a 10-L bottle under a pressure of 3.0 atm , what is the average translational kinetic energy of an oxygen molecule? Express your answer using two significant figures. Hν ΑΣφ к- J Submit Request Answer
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter16: Temperature And The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 38P
Related questions
Question

Transcribed Image Text:If 1.2 mol of oxygen gas is confined in a 10-L bottle under a pressure of 3.0 atm , what is the average translational kinetic energy of an oxygen
molecule?
Express your answer using two significant figures.
Hν ΑΣφ
к-
J
Submit
Request Answer
Expert Solution
Step 1
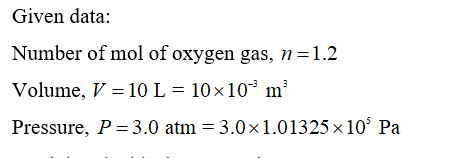
Step 2
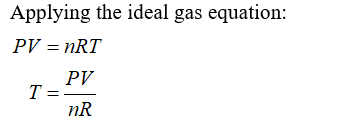
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College