
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Macmillan Learning
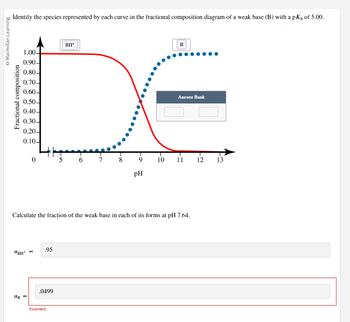
Identify the species represented by each curve in the fractional composition diagram of a weak base (B) with a pK, of 5.00.
Fractional composition
1.00-
0.90-
0.80-
0.70-
0.60-
0.50-
0.40-
0.30-
0.20-
0.10-
BH¹
ав
=
.95
.0499
BH+
Incorrect
5 6 7
00.
8
Calculate the fraction of the weak base in each of its forms at pH 7.64.
pH
10
B
Answer Bank
11 12
13
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Needing help with all questionsarrow_forwardWhich of the curves seems to have the shortest vertical line around its equivalence point? Explain why. (If you suspect that your data are inaccurate, explain which curve you think should have been most vertical and why.)arrow_forwardThe Kb for the weak base B is 7.33 x 10-7. Calculate the Ka for conjugate acid BH* at 25°C. Report your answer in scientific notation with two places past the decimal. Use this format for scientific notation: 1.23*10^-1 Type your answer....arrow_forward
- Nitesharrow_forwardHelp with the following questionarrow_forward14. If the pH at half way to the first equivalence point is 3.20, pH at first and second equivalence points of a diprotic acid are 7.20 and 13.45, respectively, which of the following is correct? a. Kaj = 7.20 %3D b. pKaj = 13.45 c. pKaj = 3.20 d. pKaj = 9.45 %3D e. pKaj = 7.20 %3Darrow_forward
- The Kg of "HA" is 4 x 10-10. Calculate the pK. (keep one decimal place). A 0.10 M solution of a weak acid, "HA*, has [Hy0+] = 0.0000072 M. Determine [A] in the solution. (Hint: Where do Hy0* and A° come from?)arrow_forwardAnswer step by steparrow_forward-9 Consider a solution that contain C-H5N and C5H5NH given Kb= 1.7 x 10-⁹ Calculate the ratio [CsHsN] [CsHsNH] if the solution has a PH= 10arrow_forward
- The pKa values of some hypothetical acids (HX, HY, HZ, HG) are shown below: HX pka=2.50, HY pKa=4.72, HZ pKa=1.87, HG pKa=5.67 Which of the following conjugate bases will be the weakest? a) X- b)Y- c)Z- d)G- e)they all have the same strength P.S could you please tell me which concept it is or a topic?arrow_forwardPlease don't provide hand written solution....arrow_forwardHadising Name: 2: Hailey Gary H3C Pre-Lab: Determination of Vinegar Acidity by Titration RES 1. Complete the following neutralization reaction. Identify the weak acid and conjugate base. O Acidic proton OH + OH + Nat Acetic Acid CHM 102arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY