
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Identify the most stable radical structure.
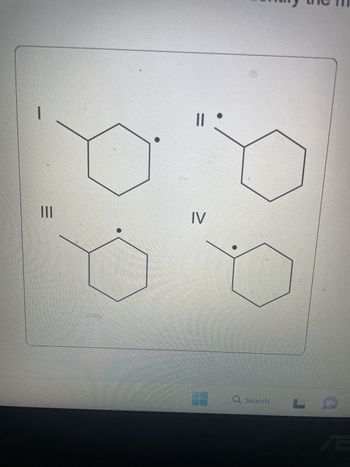
Transcribed Image Text:I
|||
ARASINA
||
IV
Q Search LG
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 13. for the following electrophilic substitution reaction, choose the resonance form that will NOT be observed during the reaction. Cl2, FeCl3 .CI CI CI .CI d) CI e) a and b a) b) c) +.arrow_forwardAssign the correct formal charge to the oxygen atom in this structure (you may right click on the atom and use the Charge menu, or use the + and - buttons on the graphical toolbar). Once the correct formal charges have been placed, identify all atoms with a reasonable formal charge by setting the map number to 1, and those with an unreasonably large formal charge by setting the map number to 2. HINT: all of the hydrogen atoms are already shown.arrow_forwardWhat is the definition of radical and show an example of an alkyl radical?arrow_forward
- Can IR spectroscopy be used for quantitative analysis? >No, because IR spectroscopy only records vibrational frequency of bonds. >No, because IR spectroscopy only identifies the types of covalent bonds. >Yes, because the transmittance of the analyte is proportional to concentration. >Yes, because the absorbance of the analyte is proportional to concentration.arrow_forward5. Good Question. Draw the best Lewis structure for each of the following common solvents. water (H₂O), methanol (CH3OH), formaldehyde (CH₂O), chloroform (CHCl3), acetonitrile (CH3CN), pentane (C5H12).arrow_forward5. Draw several resonance contributors for the cyanate ion, [OCN]. Circle the two lowest energy contributors. In the actual anion, on which atom does the negative charge primarily reside? Justify your answer.arrow_forward
- OCH Draw the molecules on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default.arrow_forwardFor each of the following pairs of resonance structures, cirele the one that would make greater contribution to the actual resonance hybrid. OCH, OCH, A- BI E =arrow_forward12. Provide one other contributing resonance form for structures A, B, and C. Show all electron movement using curved arrows. Between the two structures, circle the higher contributing resonance form. A В C HO.arrow_forward
- Two resonance structures are possible for the anion shown. One resonance form is given, but it is incomplete. Complete the given structure by adding nonbonding electrons and formal charges. Draw the remaining resonance structure, including nonbonding electrons and formal charges. Omit curved arrows. Structure A: complete the structure by adding Structure B: draw the remaining resonance structure, nonbonding electrons and formal charges. including nonbonding electrons and formal charges. Rings Erase Select Draw Rings More Erase Select Draw More H // C H. Harrow_forwardProvide the major resonance forms for the molecule shownarrow_forwardMost alkenes absorb at a shorter wavelength than alkadienes. Explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY