
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:[References)
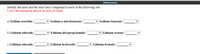
Identify the most and the least basic compound in each of the following sets.
Leave the remaining answer in each set blank.
a) Sodium acetylide:
Sodium p-nitrobenzoate:
Sodium benzoate:
b) Lithium ethoxide:
Lithium diisopropylamide:
v Lithium acetate:
c) Lithium ethoxide:
v Lithium hydroxide:
v Lithium formate:
Expert Solution
arrow_forward
Step 1
The basicity of the compound depends on several factors available. For example the electronegative atom in the structure, lone pair to donate. Generally due to loan pairs on Nitrogen are active in amide. Hence amides are a good base.
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hello, can I get help on this please? I am confused and do not understand! Thank you!arrow_forwardB). Pyridine and pyrrole (shown below) are considered to be aromatic compounds. i. Explain why this is so using orbital diagrams to illustrate your answer. and N-H ii Explain which of the two is a stronger base and explain why.arrow_forwardGive detailed Solution with explanation needed of all..don't give Handwritten answerarrow_forward
- Organic Chemistry 1 Why did you choose your choice? Explain your reasoning and rational (theory and principles and periodic trends) as to why the species is the strongest base or the strongest conjugate acid. That is to say, provide a reasoning and an answer to your selection/choice. Why did you circle your answer for that particular species?arrow_forwardFor the following compounds answer part a and b.arrow_forwardDraw the organic product of the Bronsted acid-base reaction between the compounds shown below. Include all lone pairs and charges as appropriate. Ignore any counterions. •H :F: :O: :O: H Na Ⓒ 0:0- Harrow_forward
- Give detailed Solution with explanation needed..don't give Handwritten answerarrow_forward(b) Identify the most acidic H on each molecule, then list its approximate pka in the box provided. H2N Но Но H3Narrow_forwardArrange the following molecules in increasing order of acidity. Base it only on their structural differences and explain how it is so. 1. Butanoic acid, Propionic acid, Propylamine, Butanearrow_forward
- Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each structure. The pka's for the acids of interest are: ethyl acetate(pk, -25) and ethanol(pk, 16.0) ethoxide CH₂ ethyl acetate — C ethanol a) The stronger base is b) its conjugate acid is c) The species that predominate at equilibrium are (two letters, eg. ac) D ethyl acetate enolatearrow_forwardPlease don't provide handwriting solutionarrow_forwardGive correct detailed Solution with explanation needed..please avoid handwritten Solution..please explain Huckels rulesarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY