
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
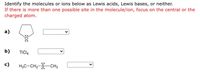
Transcribed Image Text:Identify the molecules or ions below as Lewis acids, Lewis bases, or neither.
If there is more than one possible site in the molecule/ion, focus on the central or the
charged atom.
а)
b)
TICI4
c)
H3C-CH2-S-CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the following reaction between recatant I and II, decide which reactant is the Lewis base and acid (LB/LA), and if they are also Bronsted acids/bases (BA/BB) To preview the image click here e H-CI: A B :O: + + || NH3 || 1. Choose the correct option for [Select] 2. Choose the correct option for [Select] H OH + < + :CI NH3arrow_forwardConsider the table of weak acids below. Which of the following bases be the strongest weak base? Weak A) CN- K. Acid HCN 4.9 x B) НО 10-10 H2O2 2.4 x 10-12 C) 10- HIO 2.3 x 1011 HBRO 2.5 x 10-9 D) Bro- e Type here to searcharrow_forwardIdentify the molecules or ions below as Lewis acids, Lewis bases, or neither. If there is more than one possible site in the molecule/ion, focus on the central or the charged atom. LNH2 a) b) -CH3 c) H3C-CH2-CH3arrow_forward
- HCOOH + CN HCOO + HCN Acid 中 Base No Answers Chosen No Answers Chosen Conjugate Acid Conjugate Base No Answers Chosen No Answers Chosen Possible answers CN- EHCOOH *HCN :HCOO- 来 **** **** 注arrow_forwardGive detailed Solution with explanation needed of all options with structures ..don't give Handwritten answerarrow_forward1,2arrow_forward
- Complete the table of conjugate acids and bases. [write NA if it is not applicable] Conjugate Base Conjugate Acid HSO, H2O H2CO3 NH3 5 CIO HTML Editorarrow_forwardWhich of the following are conjugate acid/base pairs? Select all that apply. Group of answer choices 1. H2SO4 and SO42- 2. NaCl and NaOH 3. H2CO3 and CO32- 4. H3PO4 and H2PO4- 5. HCl and Cl-arrow_forwardQuestion 27 of 36 Write the formula of the conjugate base of CH3CO2H. 4- 2- 2+ 3+ 4+ 1 2 3 6. 7 8. 9. 0. 1 3 4 6. 17 8. 9. 0. (s) (1) (g) (aq) CH C CHO Reset • x H2O Delete IMG-4393.jpg IMG-4392.jpg IMG-4391.jpg IMG-4390.jpg hulu P Type here to search 3.arrow_forward
- (CB,A,CA, or B) + (B,A,CB,or CA) ➡️ (B,CA,CB,or A) + (CA,A,CB,or B)arrow_forwardIdentify the molecules or ions below as Lewis acids, Lewis bases, or neither. If there is more than one possible site in the molecule/ion, focus on the central or the charged atom. NH₂ a) O b) NH3 c) H3C CH3 CH3 O (arrow_forward3 parts to this questionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY