
Introductory Chemistry For Today
8th Edition
ISBN: 9781285644561
Author: Seager
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
Identify the highest occupied molecular orbital of formaldehyde (methanal).
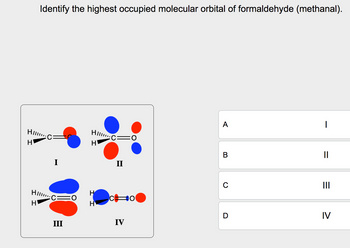
Transcribed Image Text:Identify the highest occupied molecular orbital of formaldehyde (methanal).
HIC
H
Hi
H
I
Hi..
H
A
B
II
Co
0
||
D
IV
III
IV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- H3C CN 1) i-Bu₂AIH CH3 Ph 2) H3O+arrow_forwardG.196.arrow_forward1. What type of reaction that all organic compounds undergo? 2. What is the total bond order of sulfur in CH3SCH3? 3. Explain the meaning in organic formulas of a pair of parentheses with no subscript behind it, such as in CH3CH2CH(CH3)C3H7arrow_forward
- 3. Draw the dash-wedge structure for the following molecules. 1 C₂H₂ C₂H₂ S H CH₂ 1 H Br H Cl * H H 4. Arrange the structures in the increasing order of stability CH₂ C₂H₂ C₂M₂ C₂H₂ H C₂H₂ *馬來西 H C₂H₂ H C₂H₂ CHy CH₂ IV || |||arrow_forwardIdentify A through O:arrow_forward2. Name the following molecules using IUPAC systematic naming, ignore stereochemistry. a. b. C. d. g. h. ex 8ء محكمه لار 3. Identify the hybridization (sp, sp2, sp³) of each carbon atom in the following molecules. } NH₂ 4. Identify the number of a bonds, bonds, lone pairs, and hydrogens in the following molecules. 8 1111 1111arrow_forward
- c. Which orbitals or hybrids are involved in each highlighted bond. (Carbon Sulfur double bond) . s=c=sarrow_forwardDraw curved arrows to show the movement of the electrons as the bond forms.arrow_forward6. Which of the following is a wedge and dash structure for the following Newman projection? CH3Y CH3 10: notsimotnico beroppste s to nokto H H Et on s ei privollot erts to rainW ziq S Serisqoniq HOH VI III. Et || d ||| IVarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
