
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
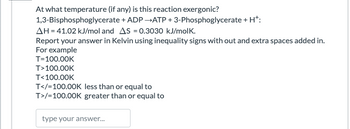
At what temperature (if any) is this reaction exergonic?
1,3-Bisphosphoglycerate + ADP →ATP + 3-Phosphoglycerate + H+:
ΔH = 41.02 kJ/mol and ΔS = 0.3030 kJ/molK.
Report your answer in Kelvin using inequality signs with out and extra spaces added in.
For example
T=100.00K
T>100.00K
T<100.00K
T</=100.00K less than or equal to
T>/=100.00K greater than or equal to
Transcribed Image Text:At what temperature (if any) is this reaction exergonic?
1,3-Bisphosphoglycerate + ADP →ATP + 3-Phosphoglycerate + H+:
AH = 41.02 kJ/mol and AS = 0.3030 kJ/molk.
Report your answer in Kelvin using inequality signs with out and extra spaces added in.
For example
T=100.00K
T>100.00K
T<100.00K
T</=100.00K less than or equal to
T>/=100.00K greater than or equal to
type your answer...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Sodium reacts violently with water according to the equation Na(s) + H2O() NaOH(aq) + H2(g) Without doing calculations, predict the signs of rH and rS for the reaction. Verify your prediction with a calculation.arrow_forwardWithout looking up their standard entropies in reference tables, identify which of the following lists the materials in order of increasing entropy. (a) H2O() NaCl(s) NH3(g) (b) H2O() NH3(g) NaCl(s) (c) NaCl(s) H2O() NH3(g) (d) NH3(g) H2O() NaCl(s)arrow_forwardAnother step in the metabolism of glucose, which occurs after the formation of glucose6-phosphate, is the conversion of fructose6-phosphate to fructose1,6-bisphosphate(bis meanstwo): Fructose6-phosphate(aq) + H2PO4(aq) fructose l,6-bisphosphate(aq) + H2O() + H+(aq) (a) This reaction has a Gibbs free energy change of +16.7 kJ/mol of fructose6-phosphate. Is it endergonic or exergonic? (b) Write the equation for the formation of 1 mol ADP fromATR for which rG = 30.5 kJ/mol. (c) Couple these two reactions to get an exergonic process;write its overall chemical equation, and calculate theGibbs free energy change.arrow_forward
- Explain why absolute entropies can be measured.arrow_forwardGiven the following information at 25C, calculate G at 25C for the reaction 2A(g)+B(g)3C(g) Substance Hf(kJ/mol) S(J/molK) A(g) 191 244 B(g) 70.8 300 C(g) 197 164 a 956 kJ b 956 kJ c 346 kJ d 346 kJ e 1.03 103 kJarrow_forwardThe enthalpy of vaporization for water is 40.65 kJ mol-1. As a design engineer for a project in a desert climate, you are exploring the option of using evaporative cooling. (a) If the air has an average volumetric heat capacity of 0.00130 J cm-3 K-1, what is the minimum mass of water that would need to evaporate in order to cool a 5 m? 5 m room with a 3 m ceiling by 5°F using this method? (b) Is this a spontaneous or nonspontaneous process?arrow_forward
- When ammonium chloride is added to water and stirred, it dissolves spontaneously and the resulting solution feels cold. Without doing any calculations, deduce the signs of G, H, and S for this process, and justify your choices.arrow_forwardPredict whether each reaction is reactant-favored or product-favored at 298 K and 1 bar, and calculate the minimum work that would have to be done to force it to occur, or the maximum work that could be done by the reaction. (a) 2 CO2(g) 2 CO(g) + O2(g) (b) 4 Fe(s) + 3 O2(g) 2 Fe2O3(s)arrow_forwardWhen a fossil fuel burns, is that fossil fuel the system? Explain your answer.arrow_forward
- For one day, keep a log of all the activities you undertake that consume Gibbs free energy. Distinguish betweenGibbs free energy provided by nutrient metabolism andthat provided by other energy resources.arrow_forwardWhat are the two ways that a final chemical state of a system can be more probable than its initial state?arrow_forwardFor the reaction BaCO3(s) BaO(s) + CO2(g), rG = +219.7 kJ/mol-rxn. Using this value and other data available in Appendix L, calculate the value of fG for BaCO3(s).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning