
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
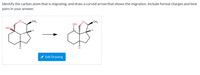
Transcribed Image Text:Identify the carbon atom that is migrating, and draw a curved arrow that shows the migration. Include formal charges and lone
pairs in your answer.
CH3
CH3
OH
НО
H
Edit Drawing
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Which molecule contains carbon with a negative formal charge? CO CO2 H2CO CH4arrow_forwardplease please answer everything i really need it ts super important answer super super fast For each molecule or ion listed below:1) Draw the complete Lewis structure, including all resonance forms.2) Count the number of bonded atoms (BA) and unshared-pairs (UP) around the central atom. Add them to get the number of electron SETS.3) Draw the molecule or ion. Include approximate bond angles on the drawing.4) State the electron set geometry and the molecular geometry.5) Build a model. If the Lewis structure shows resonance, build just one of the resonance forms.arrow_forwardA. в. с. Figure 4-8 D. СН3 0: сн. H cle 2 СН3 СН3 - н CH2 ва Бе СН3 fo: H Le :CH₂ CH 3 того CH Снзarrow_forward
- For each highlighted bond (shown in red), select the arrow that indicates the direction of bond polarity (leave the box blank for a completely nonpolar bond).arrow_forward01-7c Match the skeletal structure on the left to the line- angle structure on the left. C cccdc -C- C a) C- d) b) C=C-C-C-C-C 2) с c) C-C-c-c-cd-c 31 3) C adago C a) [Select] b) [Select] c) [Select] 1) d) [Select] 4) =+ Karrow_forward• • • • • • Draw all atoms, including hydrogen atoms. Apply formal charges where appropriate. Assign lone pairs and radical electrons where appropriate. Use the "starting points" menu to revert to the original molecule(s) shown. Draw the appropriate electron-flow arrows. Omit + signs between structures. H-0-0-0: :0-H narrow_forward
- Please help me answer CH4 SO3 SO3,2-arrow_forwardPlease don't provide handwriting solutionarrow_forward3. Keeping in mind that all row two elements need to have 8 electrons and each bond contains two electrons, draw the missing lone pair electrons on each O or N below. Then, assign the missing formal charges to each O or N. H H H H H H C H- H- C H- 中长子长 H H Harrow_forward
- What do equal signs convert to in a condensed formula ? I’m not sure if that’s a double bond. Not sure I expanded correctly and not sure how to convert the first 2 CH3’s into a Lewis without violating octet rule. Thanks for any informationarrow_forwardFor each compound, determine the direction of bond polarity. Leave the box blank for a nonpolar bond. Вr-Br H-CI F-CH, Answer Bank +arrow_forwardDraw the simplest set of curved arrows that shows how the structure on the left could be turned into the structure on the right. Show all lone pairs. If you need to expand part of the structure to show some lone pairs, expand it by drawing in all atoms and bond lines. H,N +arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Expert Answers to Latest Homework Questions
Q: I need guidance with this financial accounting problem using the right financial principles.
Q: Can you explain this general accounting question using accurate calculation methods?
Q: What is the value of ending inventory?
Q: Can you demonstrate the proper approach for solving this financial accounting question with valid…
Q: Navarro Enterprises has a beginning retained earnings balance of $78,000. Net income for the year is…
Q: General accounting
Q: I need help finding the accurate solution to this general accounting problem with valid methods.
Q: Can you explain the correct approach to solve this financial accounting question?
Q: I am looking for help with this general accounting question using proper accounting standards.
Q: Can you solve this general accounting question with accurate accounting calculations?
Q: What was Riverton supplies average collection period ?
Q: Please provide the correct solution to this financial accounting question using valid principles.
Q: hi expert provide answer please
Q: Can you provide a detailed solution to this financial accounting problem using proper principles?
Q: Please help me solve this financial accounting problem with the correct financial process.
Q: Financial accounting
Q: Complete the following reactions- hand written please
Q: Select all of the following that changes in the MC1R gene can lead to:
Changes in spots/stripes in…
Q: Pleiotropic genes are genes that (blank)
Cause a swapping of organs/structures, are the result of…
Q: A loss of function mutation in Pitx1 enhancers can cause (blank)
Removal of Pitx1 exons and growth…
Q: Hox1a most likely contributes to (blank) patterning in the developing embryo?
Ventral, posterior,…