
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
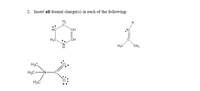
Transcribed Image Text:2. Insert all formal charge(s) in each of the following:
H2
H
HC
CH
H2Ć.
CH
H3C
CH3
H3C,
H3C-
EN-
H3C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 3. For each molecule below, draw two additional resonance reasonable structures. Be show sure to show how the resonance structures are related using formal arrow pushing. Rank the resonance structures from most stable to least stable (if they are the same stability rank them the same). Finally, draw the hybrid. ΝΗ G ygarrow_forwardAdd the missing formal chargesarrow_forwardIn which structure(s) below does the oxygen have a formal charge of -1? H-0-H H-ö: H-C=ö-H H3C-0-CH3 ČH3 I II III IV I only O Il only I and II I and IV O , II, and IV :0-Iarrow_forward
- Answer to this questionarrow_forwardFor each of the structures shown below, identify the formal charge of any atoms that are not neutral. USE IMAGE AS REFERENCEarrow_forwardANSWER THE FOLLOWING PROBLEMS AND EXPLAIN YOUR ANSWER FOR BETTER UNDERSTANDING. 1. Assign formal charges to each carbon, nitrogen and oxygen atom in the given species. H-C-H CH3-N=N: :OH || CH3-C-CH3arrow_forward
- Calculate the formal charge for each atom that is not carbon or hydrogen in the following molecules.arrow_forwardGive typed full explanation not a single word hand written otherwise leave itarrow_forward|| H-C-CH-C-H Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons and any nonzero formal charges. OF H Q Copy Answer Strings 1 H H 8arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY