
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
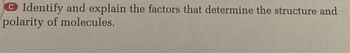
Transcribed Image Text:Identify and explain the factors that determine the structure and
polarity of molecules.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How to identify what kind of bond forms within molecules. Explain what kind of bond forms within the molecule CaCl2. Diagram how it occurs.arrow_forwardThe physical and chemical properties of a molecule depend on its structure. Here are two ball-and-stick models for two compounds that have the same molecular formula but different structures and different chemical properties. Ethanol Dimethyl etherarrow_forwardElements such as carbon, hydrogen, and oxygen that occur in biomolecules form stable covalent bonds. What would be the result if the bonds between these atoms were either slightly less or more stable than the naturally occurring bonds?arrow_forward
- What is the structure of molecule A? 1. NaNO2/ HCI A C12H10 ON2 2. Fenolarrow_forwardPhysical changes Cause a change in composition of the substances involved. Cause a change in molecular structure of the compounds involved. Cause a change in appearance of the substances involved. Cause a change in chemical bond of the substances involved.arrow_forwardCan you please help me answer thisarrow_forward
- How to identify the answer? And can you explain the concept.arrow_forwardhat is meant by the term driving forces? Why are mailer spread and energy spread considered to be driving forces?arrow_forwarda) What types of elements are bonded together to make an ionic compound? Give three examples of ionic compounds. Identify the types of elements that make up the compound in each example. B) Describe how the physical state of a substance at room temperature depends on the strength of the intermolecular forces between the particles of the substance.arrow_forward
- Draw another microscopic sketch of a small section of stream with the charged comb nearby. In your sketch, draw at least 2 layers of at least 10 water molecules. Show the polarization orientation of each water molecule in the stream of water when the charged comb is nearby. What is the distinct feature of the orientation of each molecule’s polarity in this second situation?arrow_forwardConsider the two products CO2 and H2O for the combustion of ethanol. Use what you have learned about molecular geometry, polarity, and intermolecular forces to explain why CO2 is a gas at room temperature but H2O is a liquid at room temperature.arrow_forwardTRUE if the postulate is accurate and FALSE if the postulate is flawed. Postulates 3.Collisions between molecules are perfectly elastic (that is, no energy is gained nor lost during the collision). 4.There are negligible, attractive, or repulsive forces between molecules. 5.The average kinetic energy of a molecule is constant.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning