
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
I have a sample of seawater from the open ocean with salinity S = 35.00 at 20°C. I need help finding the concentration of magnesium ion in a seawater sample at 5°C in units of mmol/kg and also in mmol/L.
Using this table, we can see that at 20 degrees Celsius, the concentration is 52.82 mmol/kg and 54.13 mM(mmol/L). The density at 5°C 1027.677 kg/m^3 and 1024.763 kg/m^3 at 20°C. The density anomaly would be 24.7896 at 20°C.
Thank you!
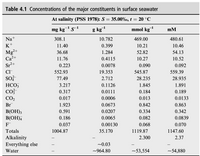
Transcribed Image Text:Table 4.1 Concentrations of the major constituents in surface seawater
At salinity (PSS 1978): S = 35.00%, t = 20 °C
mg kg¯ s-1
g kg
mmol kg
mM
Na+
308.1
10.782
469.00
480.61
K+
11.40
0.399
10.21
10.46
Mg²+
36.68
1.284
52.82
54.13
Са2+
Sr2+
CI
so?
11.76
0.4115
10.27
10.52
0.223
0.0078
0.090
0.092
552.93
19.353
545.87
559.39
77.49
2.712
28.235
28.935
НСО,
3.217
0.1126
1.845
1.891
0.317
0.0111
0.184
0.189
CO2
0.017
0.0006
0.013
0.0133
Br
1.923
0.0673
0.842
0.863
0.0207
B(OH)3
B(OH),
0.591
0.334
0.342
0.186
0.0065
0.082
0.0839
F
0.037
0.00130
0.068
0.070
Totals
1004.87
35.170
1119.87
1147.60
Alkalinity
2.300
2.37
Everything else
~0.03
Water
~964.80
~53,554
~54,880
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Similar questions
- Given that the molar mass of air is 0.029 kg mol-1, standard temperature is 0 °C and standard pressure is 101 kPa, calculate: i) the number of molecules in 0.002 m3 of air at STP. ii) the density of air at STP.arrow_forwardProblem 1: Reynold's number One can has your teeth cleaned professionally using a rotating brush or with a jet stream of a baking soda slurry. For baking soda slurries, the dentist uses a device similar to the device shown in HD Figure 8.24 to remove plaque. The fluid used in the device is a baking soda slurry. Baking soda slurry is 3.0% baking soda (sodium bicarbonate) in water, where 0.90% is the saturation limited of baking soda in water. If the inlet velocity is 0.24 m/s with inlet diameter of 5.4 mm at 23°C. The nozzle tip has a diameter of 0.51 mm. Assume no frictional effects. See page 4 for sodium bicarbonate-viscosity graph. State any other assumption needed. FIGURE 8.24 Inlet Flow Outlet Flow A. What is the Reynold's number for the fluid entering the device? B. Is the flow laminar or turbulent? C. For the same mass flow rate as in part A), determine the Reynold's number if the temperature is increased to 95°C. Hint: ratios can be used. State any assumption. Laminar or…arrow_forwardWhat volume of a 4.00x10⁻²M solution of silver nitrate contains 2x10⁻² moles of nitrate ions?arrow_forward
- 28.21 A 1 x 10-2 m spherical pellet is sprayed with a very thin coat of paint. The paint contains a volatile solvent. To dry the pellet, a 300 K and 1.013 × 105 Pa air stream flows around it with a bulk velocity of 1 m/s. The estimated loading of the solvent in the wet paint is 0.12 g solvent/cm³. Physical properties are vapor pressure of the solvent = 1.27 x 104 Pa mass diffusivity of solvent in air = 9.62 x 10-6 m²/s kinematic viscosity of air density of air = 1.569 x 10-5 m²/s = 1.177 kg/m³ thermal conductivity of air thermal diffusivity of air heat capacity of air = 2.624 x 10-2 J/m.s. K = 2.216 x 10-5 m²/s = 1.006 J/g. K = 78 g/g mole molecular weight of the solvent Use the McAdam's¹¹ equation Nu = 0.37(Redp) 0.6 (Pr) 1/3, where Redp dp vx V to evaluate (a) the heat-transfer coefficient, h; (b) the mass-transfer coefficient, ke; (c) the molar flux of the solvent into the air stream.arrow_forwardA solution is made using 250.0 mL of ethanol (density 0.7892 g/mL) and 673.1 mL of water (density 1.000 g/mL). What is the mass percent of the ethanol?arrow_forwardb) There are many examples of chemical equilibrium all around you. One example is a bottle of fizzy cooldrink. In the bottle there is carbon dioxide (CO2) dissolved in the liquid. There is also CO2 gas in the space between the liquid and the cap. There is a constant movement of CO2 from the liquid to the gas phase, and from the gas phase into the liquid. However, if you look at the bottle there does not appear to be any change. The system is in equilibrium. Write the equilibrium reaction for this event: +arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The