
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Draw the coupling product of the Pd(0)Pd(0)‑catalyzed Suzuki reaction between the compounds shown.
Show the stereochemistry of the product.
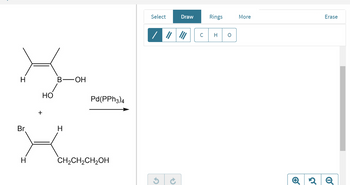
Transcribed Image Text:Br
H
+
HO
B-OH
H
Pd(PPH3)4
CH₂CH₂CH₂OH
Select
/ ||||||
G
Draw
→
Rings
C H O
More
O
Erase
O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give the organic product formed in each of the following reactions, draw a 3-D representation for the product molecule showing the correct configurationarrow_forwardThe alkene shown undergoes bromination. H (a) Draw the product(s) of bromination of this compound, including all expected stereoisomers (if any). Use wedge-and-dash bonds to designate the stereochemistry at any chirality centers, and make sure to draw an explicit hydrogen if a chirality center has one. (b) Characterize the starting alkene as having the E or Z configuration. (c) characterize the product(s). (a) H Br₂ Draw the product(s) of bromination. Br H Brarrow_forwardChoose the right reagent or series of reagents from the ones listed to synthesize the following molecule (see picture) from benzene (C6H₁) or toluene (C6H5 CH₂). COOH NH₂ C6H₁ and HNO3, H₂SO4 followed by CH3 Cl, AlCl3 then KMnO4, A ○ C₁H₂CH3 and KMnO4, A, followed by HNO3, H₂SO4 ○ C6H₂ CH3 and HNO3, H₂SO4, followed by KMnO4, A, then Zn, HCI C6H₁ and CH3OH followed by KMnO4, A, then HNO3, H₂SO4 C6H₁ CH3 and HNO3, H₂SO4, followed by Zn, HC1arrow_forward
- How is the mechanism for oxymercuration/demercuration reactions of alkenes understood? How are the (Markovnikov) products predicted and what reagents would you need to use to incorporate this reaction into a multistep synthesis strategy. Finally, what makes this a potentially more useful way to synthesize alcohols from alkenes than simple acid hydration?arrow_forwardgive the major products of this reaction. include stereochemistry as appropiate. strongly appreciate it.arrow_forwardProvide the systematic name of the major product after (S)-1-bromo-4-methylhexane reacts with sodium hydroxide in diethyl ether.arrow_forward
- Predict the stereochemistry for the following E2 reaction. Draw a Newmann Projection of the reactive conformation and the structure for only major product of the reactionarrow_forwardDraw an alkyl halide that would undergo an SN2 reaction to yield this product under the conditions shown below. Use a dash or wedge bond to indicate the stereochemistry of substituents on asymmetric centers. Ignore inorganic byproducts. S Drawing NaSCH THEarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY