
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
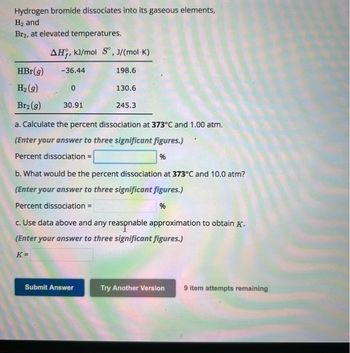
Transcribed Image Text:Hydrogen bromide dissociates into its gaseous elements,
H₂ and
Br2, at elevated temperatures.
HBr(g)
H₂(g)
Br₂(g)
AH, kJ/mol S, J/(mol-K)
-36.44
0
30.91
198.6
130.6
245.3
a. Calculate the percent dissociation at 373°C and 1.00 atm.
(Enter your answer to three significant figures.)
Percent dissociation =
%
Submit Answer
b. What would be the percent dissociation at 373°C and 10.0 atm?
(Enter your answer to three significant figures.)
Percent dissociation =
%
c. Use data above and any reasonable approximation to obtain K.
(Enter your answer to three significant figures.)
K=
Try Another Version
9 item attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider a metal ion A2+ and its nitrate salt, In an experiment, 35.00 mL of a 0.217 M solution of A(NO3)2 is made to react with 25.00 mL of 0.195 M NaOH. A precipitate, A(OH)2, forms. Along with the precipitation, the temperature increases from 24.8C to 28.2C. What is H for the precipitation of A(OH)2? The following assumptions can be made. • The density of the solution is 1.00 g/mL. • Volumes are additive. • The specific heat of the solution is 4.18 J/g C.arrow_forwardReword the statement in Question 109 so that it is always true. Criticize this statement: Provided it occurs at an appreciable rate, any chemical reaction for which rG 0 will proceed until all reactants have been converted toproducts.arrow_forwardConsider the reaction CO(g)+H2O(g)CO2(g)+H2(g) Use the appropriate tables to calculate (a) G at 552C (b) K at 552Carrow_forward
- Calculate H when a 38-g sample of glucose, C6H12O6(s), burns in excess O2(g) to form CO2(g) and H2O() in a reaction at constant pressure and 298.15 K.arrow_forwardExplain how the entropy of the universe increases when an aluminum metal can is made from aluminum ore. Thefirst step is to extract the ore, which is primarily a formof A12O3, from the ground. After it is purified by freeingit from oxides of silicon and iron, aluminum oxide ischanged to the metal by an input of electrical energy. 2Al2O3(s)electricalenergy4Al(s)+3O2(g)arrow_forwardWhen 7.11 g NH4NO3 is added to 100 mL water, the temperature of the calorimeter contents decreases from 22.1 C to 17.1 C. Assuming that the mixture has the same specific heat as water and a mass of 107 g, calculate the heat q. Is the dissolution of ammonium nitrate exothermic or endothermic?arrow_forward
- Silver carbonate, Ag2CO3, is a light yellow compound that decomposes when heated to give silver oxide and carbon dioxide: Ag2CO3(s)Ag2O(s)+CO2(g) A researcher measured the partial pressure of carbon dioxide over a sample of silver carbonate at 220C and found that it was 1.37 atm. Calculate the partial pressure of carbon dioxide at 25C. The standard enthalpies of formation of silver carbonate and silver oxide at 25C are 505.9 kJ/mol and 31.05 kJ/mol, respectively. Make any reasonable assumptions in your calculations. State the assumptions that you make, and note why you think they are reasonable.arrow_forwardPredict whether each of the following processes results in an increase in entropy in the system. (Define reactants and products as the system.) (a) Water vapor condenses to liquid water at 90 C and 1 atm pressure. (b) The exothermic reaction of Na(s) and Cl2(g) forms NaCl(s). (c) The endothermic reaction of H2 and I2 produces an equilibrium mixture of H2(g), I2(g), and HI(g). (d) Solid NaCl dissolves in water forming a saturated solution.arrow_forward5.12. True or false: If all the partial pressures of reactants and products drop by half, the value of Q drops by half. Give an example of a chemical reaction to support your answer.arrow_forward
- Classify the following processes as exergonic or endergonic. Explain your answers. a.An automobile being pushed up a slight hill from point of view of the pushing b.Ice melting from point of view of the ice c.Ice melting from point of view of surrounding of the ice d.Steam condensing to liquid water from point of view of the steam e.Steam condensing to liquid water from point of view of surrounding of the steamarrow_forwardDescribe a nonchemical system that is not in equilibrium, and explain why equilibrium has not been achieved.arrow_forwardConsider the reaction NH4+(aq) H+(aq)+NH3(aq) Use G f for NH3(aq) at 25C=26.7 kJ/mol and the appropriate tables to calculate (a) G at 25C (b) Ka at 25Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co