
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
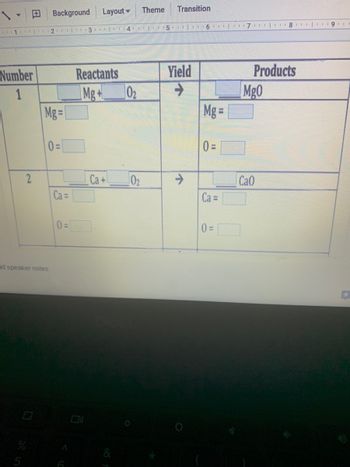
Transcribed Image Text:Background Layout
Theme Transition
11|2|3|4|56|17|T
Number
Reactants
Yield
Products
1
2
Mg=
0=
did speaker notes
Ca=
0=
Mg+ 0₂
Ca+
&
↑
Mg =
0=
Ca =
0=
Mg0
CaO
891
MASHAR
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hi! Can you be so kind to help me do #5,#6,#8 and #10. Thanks!arrow_forwardPart A Na +I2 Express your answer as a balanced chemical equation. ? ΑΣφ Request Answer Previous Answers Submit X Incorrect; Try Again; One attempt remaining Part B MacBook Ai tarrow_forwardGiven the reaction: 2 Na(s) + Cl2(g) → 2 NaCI(s) Which statement is true? O The conversion factor for chlorine gas to sodium chloride is: 1 mol Cl2 is equivalent to 2 mol NaCI O The conversion factor for chlorine gas to sodium chloride is: 2 mol Cl is equivalent to 2 mol NaCl O The conversion factor for sodium metal to sodium chloride is: 1 mol Na is equivalent to 2 mol NaCl O The conversion factor for sodium metal to sodium chloride is: 2 mol Na is equivalent to 1 mol NaCIarrow_forward
- Predict the chemical formulas of the compounds formed by the following pairs of ions.arrow_forwardEx. 110 - Calculating Mass of 3 attempts left Check my work What mass of oxygen is needed to react with 1.79 gal of methanol according to the balanced equation below? (1.00 gal = 3.79 L, and the density of methanol is 0.793 g/mL.) 2 CH3OH() + 3 02g) – 2 CO2g) + 4 H2O(g) If appropriate, express your answer in scientific notion. (Click on the answer box to show the pallet.) < Prev 3 of 5 ***** ****arrow_forwardHow can we find the percentage? And the produced?arrow_forward
- Nonearrow_forwardFumaric bromination 2.5 mL 10% bromine was added to 0.200g of fumaric acid. Calculate the limiting reagent and % yield of the reaction. NB: use density to find the mass of bromine.arrow_forwardQ) A student misreads the directions and adds 4.0 g of benzoic acid instead of 0.4 g. What do you expect to happen? Would the experiment still be valid? Explain your reasoning. Pure Lauric Acid Lauric Acid + 0.4 g Benzoic Acid Lauric Acid + 0.5 g Benzoic Acid Mass of Lauric Acid 3.03g 3.00g 3.00g Mass of Benzoic Acid 0 g 0.40g 0.50g Freezing Point 43’C 39’C 38’C Freezing Point Depression * 4’C 5’C Molality of Benzoic Acid in Lauric Acid* 1.026 mol/kg 1.282 mol/kg Moles of Benzoic Acid * 0.003 moles 0.004 moles Experimental Molar Mass of Benzoic Acid * 133.3g/mol 125 g/mol Average Molar Mass * 129.15g/mol Percent Error * 5.75%arrow_forward
- 0.10 M formic acid (HCHO₂) Ka = 1.8 x 104 0.10 M sodium formate (NaCHO₂) 0.10 M acetic acid (HC2H302) Ka = 1.8 x 10¹5 0.10 M sodium acetate (Na C₂H30₂) 0.10 M carbonic acid (H₂CO3) Ka = 4.3 x 107 0.10 M sodium bicarbonate (NaHCO3) 0.10 M KOH 0.10 M HCI 0.10 M boric acid (H3BO3) Ka = 5.8 x 10-10 0.10 M sodium borate (NaH₂BO3) 4. Which reagents listed above should be mixed to result in a buffer of about pH 6-8?arrow_forwardAll the following reactions follow the law of conservation of mass except--- 4CO2 + 2H2O 2Fe2O3 2C2H2 +502 4Fe +302 NaHCO3 Na2CO3 + H2O + CO2 2CH3OH + 302 2CO2 + 4H₂Oarrow_forwardAnswered -Correct! U8HW Question 15 == Homework 11:59 PM Answered Due Mar 19th, Resubmit Reacting water with magnesium nitride produces ammonia (NH3) and magnesium hydroxide. If this process is 70% efficient, what mass of ammonia (in kg) can be prepared from 19.5 kg of magnesium nitride? Type your numeric answer and submit 2.28 Answered - Incorrect U8HW Question 16 K Answered Due Mar 19th, == Homework 11:59 PM ⭑ You are incorrect Resubm Open in Readingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY