
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Label the most acidic hydrogen in each molecule and justify your choice by using appropriate pKa values
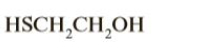
Transcribed Image Text:HSCH,CH,OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 11. Draw a circle around the most acidic hydrogen(s) and a square around the least acidic hydrogen (s)of the following compound. Explain the reason for your answer. Do not say because of their pKa values! You need to to_use ARIO to explain the reason underlying your choices Но ОН CH-CH H2C-CH3 OHarrow_forwardOrganic Chemistry 1 Why did you choose your choice? Explain your reasoning and rational (theory and principles and periodic trends) as to why the species is the strongest base or the strongest conjugate acid. That is to say, provide a reasoning and an answer to your selection/choice. Why did you circle your answer for that particular species?arrow_forwardAnswer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each structure. A table of pka values for various organic and inorganic acids can be found in the references section. ata CH3 CH3 -CH3 CH3 CH,- в D ethoxide ethanol dimethylsulfone anion dimethylsulfone a) The weaker base is b) Its conjugate acid is c) The species that predominate at equilibrium are (two letters, e.g. AC)arrow_forward
- (b) Identify the most acidic H on each molecule, then list its approximate pka in the box provided. H2N Но Но H3Narrow_forwardAnswer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each structure. A table of pK₂ values for various organic and inorganic acids can be found in the references section. OH hydroxide + CH3 H₂ NO₂ nitroethane HOH C water + a) The weaker base is b) Its conjugate acid is c) The species that predominate at equilibrium are (two letters, e.g. AC) CH₂ D nitroethane anionarrow_forward25arrow_forward
- 1. Rank the acidity of the hydrogen atoms shown on the following molecules (1= most acidic, 4=least acidic). Be sure to provide the reasoning for your choices. H H H. A Rank: 1. 2. 3. 4. Reasoning: o= 0=arrow_forwardAnswer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each structure.arrow_forward4. Arrange the compounds in the following set in order of increasing basicity (1-the most basic; 3-the least basic). Explain your answer! Magnesium hydroxide, magnesium acetate, methylmagnesium bromidearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY