
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
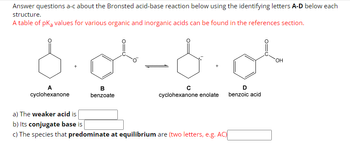
Transcribed Image Text:Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each
structure.
A table of pKa values for various organic and inorganic acids can be found in the references section.
-d.ol
A
cyclohexanone
B
benzoate
cyclohexanone enolate
a) The weaker acid is
b) Its conjugate base is
c) The species that predominate at equilibrium are (two letters, e.g. AC)
D
benzoic acid
OH
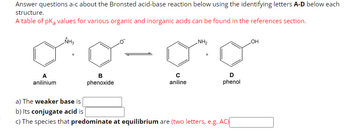
Transcribed Image Text:Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each
structure.
A table of pKa values for various organic and inorganic acids can be found in the references section.
A
anilinium
NH3
+
B
phenoxide
с
aniline
NH₂
D
phenol
a) The weaker base is
b) Its conjugate acid is
c) The species that predominate at equilibrium are (two letters, e.g. AC)
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which is true regarding the direction of the following reaction? CH3COOH (aq) + H2PO-4 <<>>> CH3COO- + H3PO4 a) the reaction favors the reactant side b) the reaction favors the product side c) the reaction favors both reactants and products equally d) the table of acidity does not proviede enough information to answer this questionarrow_forwardGive detailed Solution with explanation neededarrow_forwardPrepare CH3COOC2H5, ethyl acetate, using ethyl alcohol. Write the chemical reaction. The pain reliever acetaminophen is produced by reacting 4-aminophenol with acetic anhydride. Outline a synthesis of acetaminophen from 4-aminophenol including any needed inorganic reagents.arrow_forward
- To test a spectrophotometer’s accuracy, a solution of 60.06 ppm K2Cr2O7 is prepared and analyzed. This solution has an expected absorbance of 0.641 at 350.0 nm. Several aliquots of the solution produce the following absorbance values. 0.640 0.638 0.640 0.639 0.640 0.639 0.638 is there any significant difference between the experimental mean and the expected value at a 99.9% confidence level? Question 21 options: Yes because tcal<tcritical Yes because tcal>tcritical No because tcal<tcritical No because tcal>tcriticalarrow_forwardDraw the major organic product of the Bronsted acid-base reaction. Include all lone pairs and charges as appropriate. Ignore any counterions. I :O: NaOH :O: Select to Draw Harrow_forward[References] Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each structure. The pKa's for the acids of interest are: acetic acid (pK, 4.8), and hydrogen bromide (pK,=-9). CH3 Br CH3 H-Br OH B acetic acid bromide acetate hydrogen bromide a) The stronger base is b) Its conjugate acid is c) The species that predominate at equilibrium are (two letters, e.g. ac) Submit Answer Try Another Version 1 item attempt remaining Visited Previous Nex Email Instructor Sav Cengage Learning | Cengage Technical Supportarrow_forward
- 4) Which direction is favored when dissolved in acetone? Does the answer change if dissolved in methanol? Explain. Nal cearrow_forwardOrganic Chemistry 1 Why did you choose your choice? Explain your reasoning and rational (theory and principles and periodic trends) as to why the species is the strongest base or the strongest conjugate acid. That is to say, provide a reasoning and an answer to your selection/choice. Why did you circle your answer for that particular species?arrow_forwardPlease correct these within the same method provided. no stick form i dont like those. i attached the example i dont NOT WANT IT. SO PLEASE DONT USE IT.arrow_forward
- Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each structure. A table of pka values for various organic and inorganic acids can be found in the references section. ata CH3 CH3 -CH3 CH3 CH,- в D ethoxide ethanol dimethylsulfone anion dimethylsulfone a) The weaker base is b) Its conjugate acid is c) The species that predominate at equilibrium are (two letters, e.g. AC)arrow_forward3.) Based on the structure of its conjugate base, which acid has the lowest pKa? A. CH3COOH B. CICH2COOH C. BRCH2COOH D. CICH2CHCOOH Keywords: pKa, acid strength, dissociation, conjugate base, elemental effects, inductive-electron withdrawal, hybridization, resonance, electronegativity, proximity Concepts: structural implications for conjugate-base stabilityarrow_forwardHelparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY