
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
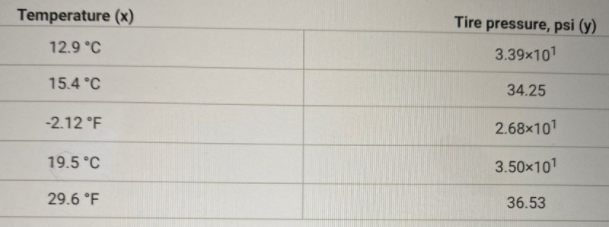
How would I find the slope between temp and tire pressure ? What would it be?

Transcribed Image Text:Temperature (x)
Tire pressure, psi (y)
12.9 °C
3.39x101
15.4 °C
34.25
-2.12 °F
2.68x101
19.5 °C
3.50x101
29.6 °F
36.53
Expert Solution
arrow_forward
Step 1
The data of pressure v/s temperature is given as,
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Similar questions
- A 0.116 mol sample of helium gas has a volume of 981 milliliters at a pressure of 4.85 atm. The temperature of the He gas sample on the Celsius scale is ______°C.arrow_forwardWould a human body float in the dead sea?arrow_forwardWhat is the density of CH3(g) (molecular mass of 15.04 g/mol) at 4.0 atm and 288 K? (Hint: assume 1 mole of gas) Give your answer to 2 decimal places and no units.arrow_forward
- When solving through a gas stoichiometry problem, you want to begin with PV=nRT if a grams is what you are ultimately solving for. b volume is what you are ultimately solving for. c temperature is what you are ultimately solving for. d pressure is what you are ultimately solving for.arrow_forwardaverage speed?arrow_forwardConsider a manometer (a barometer-like device used for measuring pressure) constructed using ethyl alcohol (ρ =0.789 g/mL). What would be the column height if the pressure is 31 mm Hg? The density of Hg is 13.56 g/mL) h = ____ cmarrow_forward
- A force of 2.31 * 104 N is applied to a diver’s face mask that has an area of 125 cm2. Find the pressure in atm on the face mask.arrow_forwardA balloon is blown up to a volume of 0.55 L at 71 °F. The balloon sits in a hot car, which reaches 107 °F. Part A Assuming constant pressure, what is the new volume of the balloon? Express your answer to two significant figures and include the appropriate units. μΑ Units Value Submit Request Answer P Pearson Copyright 2019 Pearson Education Inc. All rights reserved. | Terms of Use Privacy Poli ery TNEL TUI 1C I TIJCT Only 2 left in stock - order soon. tarrow_forwardA pipet delivers 9.97 g of water at 19°C.What volume does the pipet deliver?arrow_forward
- Question 2 of 21 Submit Convert 797 mm Hg to atm STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 1.01325 1 1.05 760 1000 6.06 x 105 101.325 14.70 105 7.97 797 0.98692 1.01325 x 105 0.001 atm kPa psi torr mm Hg Pa bararrow_forwardDescription You will use an online simulation to manipulate and calculate the four properties of gases: Pressure, Volume, Quantity, and Temperature. You will also apply your knowledge to several scenarios. To begin, open the simulation, Gases Intro, from https://phet.colorado.edu/sims/html/gases-intro/latest/gases-intro en.html. This will open it in another window. Select the simulation box Intro at the bottom. When it opens, check Width to show the width of the box. You should leave the rest unchecked. We will only use Heavy Particles. Play around with the simulation to see how to adjust the volume/width (handle on left of box), temperature (bucket below with either ice or fire), and quantity (using the pump handle to right of the box). Keep the box sealed by keeping top door closed. When you are ready to do the experiments, reset the simulation by pressing the white eraser button on bottom right, make sure width is 10.0 nm, and set to heavy particles (purple gas molecules).…arrow_forwardReview & Preview Drag and drop the appropriate conversions to solve the following problem: 1 mile 1 foot 5280 feet 12 inches 5280 feet 12 inches 1 mile 1 foot 10 km You're going to run a "10k" next month. A "10k" is a distance of 10 kilometers. How many miles will you run? You'll need these: 1000 m 1 km 1 km 1000 m 1 m 100 cm 100 cm 1 m 2.54 cm 1 inch 1 inch 2.54 cm milesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY