
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
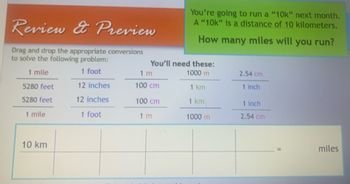
Transcribed Image Text:Review & Preview
Drag and drop the appropriate conversions
to solve the following problem:
1 mile
1 foot
5280 feet
12 inches
5280 feet
12 inches
1 mile
1 foot
10 km
You're going to run a "10k" next month.
A "10k" is a distance of 10 kilometers.
How many miles will you run?
You'll need these:
1000 m
1 km
1 km
1000 m
1 m
100 cm
100 cm
1 m
2.54 cm
1 inch
1 inch
2.54 cm
miles
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Countinly Multiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 7.808 cm x 2.2 cm 20.947 x 45. L = mol L 419.9 m+ 83.888 s Explanation = Check U cm mol m 0-- OP X S Ⓒ2022 McGraw Hill CDIarrow_forwardPlease don't provide handwriting solutionarrow_forwardMultiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 400.6 g 44.80 mL = 2.09476 mol L x 4.30 L = Expnation 0 Check U 09 mL m 869.8 m 0.29 s = 0 S mol X Ⓒ2023 McGraw Hill LLC AB Rights Reservedarrow_forward
- For the laboratory equipment below give me the name, what it is used for, minimum measurement in mL, maximum measurement in mL, smallest incremental measurement in mL, and the uncertainty in percentage when at maximum measurement.arrow_forwardNot sure what I am doing wrong here...arrow_forwardI need help with my Chemistry problems. Can you help me, please?arrow_forward
- Add or subtract the following measurements. Be sure each answer you enter contains the correct number of significant digits. 8.60 mL + 17.827 mL = 14.627 mL +5.7 mL 9.57 mL 1.8 mL = 0 mL mL mL □ x10arrow_forwardGiven the equalities below and the metric relationships you are supposed to know, convert 1.60 x 104 mL to quarts (qt) using dimensional analysis.arrow_forwardA 17.0-cm-long cylindrical glass tube, sealed at one end, is filled with ethanol. The mass of ethanol needed to fill the tube is found to be 49.23 g. The density of ethanol is 0.789 g/mL. Calculate the inner diameter of the tube in centimeters. Express your answer in centimeters to three significant figures. diameter = Submit 15| ΑΣΦ Request Answer ? cmarrow_forward
- ch 2arrow_forwardThe table below lists several conversion factors that may be useful for this problem: Conversion Factors 2 bananas = 3 carrots 2 apples = 5 oranges 7 oranges = 6 figs Chad has a strange units system for his time machine. He figures his time machine can travel 6 apples per banana. What would this be in oranges per carrot? I need help with this question. Can you please show step by step where each number goes and why. Please and thank you.arrow_forwardPlease help me! Why is is it wrong and what is the correct answer?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY