
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
How were the enthalpies found on the graph? H(f) = 10 btu/lbm (5% H2SO4 at 60 degrees F)
H(l) = -17 btu/lbm (40% H2SO4 at 180 degrees F)
![PDF *Elementary Principles of Chemic X +
74°F
Sunny
File | E:/Elementary%20Principles%20of%20Chemical%20Processes,%204th%20Edition%20(%20PDFDrive%20).pdf
Draw
T Read aloud
From Figure 8.5-1:
Energy Balance:
Q Search
This applies,
(to example8.5=3
Fusion
farmase
469 of 695
Ⓡ
Ciliquid phase-
The enthalpy of water vapor at 180°F and 1 atm relative to liquid water at 32°F may be obtained from the
steam tables in Perry's Chemical Engineers' Handbook (see Footnote 5) as
vivapur phase
=
HF 10 Btu/lbm
H=-17 Btu/lbm
m₁(lbm)
XA1 (lbm A/lbm)
A₁(Btu/lbm)
m₂(lbm)
XA2(lbm A/lbm)
Ĥ₂(Btu/lb)
OD
Hv 1138 Btu/lbm
Q = AH = m₂ Ay+ m₂A₁-(1000 lbm/h)ĤF
= [(875)(1138) + (125)(-17) - (1000)(10)] Btu/h
984,000 Btu/h/
■
Compare the ease of this computation with that of Example 8.5-1. Having the enthalpy-concentration
chart eliminates the need for all of the hypothetical heating, cooling, and isothermal mixing steps that would
normally be required to evaluate the total enthalpy change for the process.
Processes involving adiabatic mixing are particularly simple to analyze when an H-x chart is
available. Suppose XÃ is the mass fraction of A in a mixture of two species, A and B and that a
mass m₁ of Solution 1 (XA1, Ĥ₁) is/mixed adiabatically with a mass m₂ of Solution 2) (XA2, H₂) We
will show that the condition of the product mixture, (xÃ3, Ħ3), is on a straight line on the H-x chart
between the points corresponding to the feed stream conditions.
(5% H₂SO4 at 60°E)
(40% H₂SO4 at 180°F)
m3(lbm)
XA3(lbm A/lbm)
A3(Btu/lbm)
8.5 Mixing and Solution 449
Ĥ₂
Ĥ₂
Ĥ₁
ΚΑΙ
XA3
XA3
XA2
m₁xAl + m₂xA2
m₁ + m₂
{"
Ⓡ
J
63
50
D
ENG
Sign in
I
00
+
O
8:43 PM
5/21/2023](https://content.bartleby.com/qna-images/question/9ba88ecd-654b-4f6b-9783-e93c33ca6a16/c1edba97-8b40-40d2-a20b-a65739c1baa6/vwh27d_thumbnail.png)
Transcribed Image Text:PDF *Elementary Principles of Chemic X +
74°F
Sunny
File | E:/Elementary%20Principles%20of%20Chemical%20Processes,%204th%20Edition%20(%20PDFDrive%20).pdf
Draw
T Read aloud
From Figure 8.5-1:
Energy Balance:
Q Search
This applies,
(to example8.5=3
Fusion
farmase
469 of 695
Ⓡ
Ciliquid phase-
The enthalpy of water vapor at 180°F and 1 atm relative to liquid water at 32°F may be obtained from the
steam tables in Perry's Chemical Engineers' Handbook (see Footnote 5) as
vivapur phase
=
HF 10 Btu/lbm
H=-17 Btu/lbm
m₁(lbm)
XA1 (lbm A/lbm)
A₁(Btu/lbm)
m₂(lbm)
XA2(lbm A/lbm)
Ĥ₂(Btu/lb)
OD
Hv 1138 Btu/lbm
Q = AH = m₂ Ay+ m₂A₁-(1000 lbm/h)ĤF
= [(875)(1138) + (125)(-17) - (1000)(10)] Btu/h
984,000 Btu/h/
■
Compare the ease of this computation with that of Example 8.5-1. Having the enthalpy-concentration
chart eliminates the need for all of the hypothetical heating, cooling, and isothermal mixing steps that would
normally be required to evaluate the total enthalpy change for the process.
Processes involving adiabatic mixing are particularly simple to analyze when an H-x chart is
available. Suppose XÃ is the mass fraction of A in a mixture of two species, A and B and that a
mass m₁ of Solution 1 (XA1, Ĥ₁) is/mixed adiabatically with a mass m₂ of Solution 2) (XA2, H₂) We
will show that the condition of the product mixture, (xÃ3, Ħ3), is on a straight line on the H-x chart
between the points corresponding to the feed stream conditions.
(5% H₂SO4 at 60°E)
(40% H₂SO4 at 180°F)
m3(lbm)
XA3(lbm A/lbm)
A3(Btu/lbm)
8.5 Mixing and Solution 449
Ĥ₂
Ĥ₂
Ĥ₁
ΚΑΙ
XA3
XA3
XA2
m₁xAl + m₂xA2
m₁ + m₂
{"
Ⓡ
J
63
50
D
ENG
Sign in
I
00
+
O
8:43 PM
5/21/2023
Transcribed Image Text:PDF *Elementary Principles of Chemic X
59°F
Sunny
YouTube
Draw
File | E:/Elementary%20Principles%20of%20Chemical%20Processes,%204th%20Edition%20(%20PDFDrive%20).pdf
X +
T Read aloud
(Btu/lb solution)
Q Search
140
120
100
80
60
40
20
0
-20
-40
-60
-80
-100
-120
-140
467 of 695
0 0.10 0.20
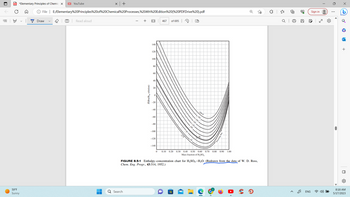
FIGURE 8.5-1 Enthalpy-concentration
Chem. Eng. Progr., 43:314, 1952.)
32°F
60
100
40
CD
140
120
200°F
180
160
Freezing line
0.30 0.40 0.50 0.60 0.70
Mass fraction of H₂SO4
chart for H₂SO4-H₂O. (Redrawn from the data of W. D. Ross,
0.80
0.90 1.00
{"
J
63
50
D
ENG
Sign in
(0)
+
O
8:18 AM
5/17/2023
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- I need this ASAParrow_forwardAn unknown compound is analyzed and found to contain 0.1935 g of carbon, 0.0325 g of hydrogen, and 0.2043 g of fluorine.The molar mass of the compound is 240.23 g/mol. a) How many moles of carbon are present in the compound? b) How many moles of hydrogen are present in the compound? c) How many moles of fluorine are present in the compound? d) Determine the empirical formula of the unknown compound. e) What is the molar mass of the empirical formula? f) What is the molecular formula of the unknown compound?arrow_forwardUsing the provided ternary phase diagram 1. What is the number of phases in this liquid mixture? Liquid Amount || Amount (mol) A || 15 B || 20 C || 7.5 xT || xW || xE 0.5 || 0.25 || 0.25 0.33 ||0.33 || 0.33 0.1 || 0.8 || 0.2 2. What are the phase compositions of the mixtures in Problem 1? What are the trichloromethane-rich and water-rich phases' compositions if there are two phases?arrow_forward
- 4. Adsorption Isotherm Curve: The low pressure (beginning region) of an adsorption isotherm curve can be approximated with a linear equation of the form: X₁* = K x P₁. The data in the table below provides laboratory measured values of the partial pressure of an adsorbate P¡ versus the mass fraction of adsorbate on the adsorbent X;*. Determine the value of K for the equation above using the data in the table, and also provide the r-squared value of this linear equation fit. Recommend you use the linear regression function in EXCEL to determine these answers. X* (gr adsorbate/gr PCC14 (mm Hg) adsorbent) 0 0 2 0.084 3.5 0.15 5 0.27 7 0.38 10 0.525 Value of K in equation above r-squared value from linear equation fitarrow_forwardCalculate Npr = ______x10^3 Please show your complete solution. When solving write the units. Please write clearly and readable. Thank you.arrow_forwardplease answer question 3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The