
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:C? Add the e
to: (1) heatin
melting the ie
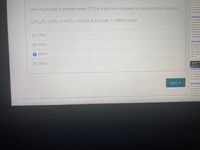
How much heat is evolved when 27.0 g of glucose is burned according to this equation?
water from 0
Evaporating
physics
1. a) using h-
C6H1206 + 602→6CO2+ 6H2O; A H comb. = -2808 kJ/mol
%3D
conservation
determine th
of fusion for
percentage e
determined v
O 136kJ
-0.1kg initial
- 56 C Melted
Chemist
O 421kJ
How much he
converting 1.
155.0 C to ic
O 280kJ
heat capacity
J/(g C) and
Jg C).
O 241kJ
You can break
violence
Jut aday can
Aghting ringi
Chemist
How do I calo
heat needed
at -24 degrees
28 degrees ce
Next
Chemist
Two 20.0-g ice
are placed into
25.0 "C. Assun
transferred to
surroundings,
Net caued
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 18 of 39 Submit In the following reaction, how many moles of CH3OH are required to give off 5576 kJ of heat? 2 CH,OH (I) + 3 O2 (g) → 2 CO2 (g) + 4 H¿O(g) AH° = -1280. kJ mol 1 2 3 4 C 7 +/- x 10 0 LO 00arrow_forwardThe following thermochemical equation is for the reaction of carbon monoxide(g) with oxygen(g) to form carbon dioxide(g). 2CO(g) + O2(g)- →2CO2(g) AH=-566 kJ How many grams of CO(g) would have to react to produce 104 kJ of energy? gramsarrow_forwardIn the following reaction, how much heat is generated when 3.21 moles of CH4 are burned? CH4 (g) + 2 O2 (g) → CO2 (g) + 2 H2O(g) AH° = -802 kJ/molarrow_forward
- Consider the following reaction carried out under constant pressure 5Cl2(g) + 2P(s) → 2PCl5(g) Δ Hrxn = -749.8 kJCalculate the heat associated with the complete reaction of 84.0 g of Cl2 with 20.6 g of P. -6.15×102 kJ -1.78×102 kJ -4.99×102 kJ -8.88×102 kJ -2.50×102 kJarrow_forward4. Consider the equations for these reactions: % N2 + % O2 → NO NO2 → NO + % 02 AH = +90.4 kJ AH = +56.5 All reactants are gaseous. What is the heat of formation (AH;) for NO2 gas?arrow_forwardThe heat of reaction for the combustion of benzene (C6H6) is –3909.9 kJ. 2 C6H6 15 O2 → 12 CO2 + 6 H20 What would be the heat change when 161.54 g of benzene is burned in kilojoules?arrow_forward
- 2. A solid substance has a mass of 250 grams. It is cooled by 25.00°C and loses 4.937 kJ of heat. What is the specific heat capacity of the substance? 20 3. A 10.0 g sample of pure acetic acid, CH3CO2H, is completely burned. The heat released warms 2.0 L of water from 22.3°C to 39.6°C. I MAR a) Assuming that no heat was lost to the calorimeter, what is the molar enthalpy change of the complete combustion of acetic acid? Express your answer in kJ/mol. b) Will the bonds in the products be longer or shorter than the bonds in the reactants?arrow_forwardWhich is not a factor of an endothermic reaction? OAHIS negative. O AH is on the reactant (left) side of the reaction. O Heat is absorbed. O AH is positive.arrow_forwardA certain reaction has Ho = -43.40 kJ and So = 23.20 J/K.(a) Is this reaction exothermic, endothermic or isothermic (neither)?This reaction is________ .(b) Does this reaction lead to a decrease, an increase, or no change in the degree of disorder in the system?This reaction leads to_______ in the disorder of the system.arrow_forward
- Use the References to access important values if needed for this question. When a solid dissolves in water, heat may be evolved or absorbed. The heat of dissolution (dissolving) can be determined using a coffee cup calorimeter. Thermometer Cardboard or In the laboratory a general chemistry student finds that when 14.72 g of BaBr2(s) are Styrofoam lid dissolved in 101.00 g of water, the temperature of the solution increases from 22.80 to 25.27 °C. The heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.82 J/°C. Nested Styrofoam cups Based on the student's observation, calculate the enthalpy of dissolution of BaBr2(s) in kJ/mol. Reaction occurs in solution. Assume the specific heat of the solution is equal to the specific heat of water. AHdissolution = kJ/molarrow_forwardThe overall reaction in commercial heat packs can be represented as 4Fe(s) + 302(g) →2Fe₂O3 (8) AH = -1652 kJ a. How much heat is released when 2.20 mol iron is reacted with excess O₂? kl released b. How much heat is released when 1.83 mol Fe2O3 is produced? kJ released c. How much heat is released when 2.20 g iron is reacted with excess O₂? kJ released d. How much heat is released when 11.8 g Fe and 2.61 g O2 are reacted? Submit Answer [Review Topics] kl released Retry Entire Group [References] 8 more group attempts remainingarrow_forwardAn amount of 10.24 kcal of heat is added to a 35.0g sample of a liquid at 25.00 °C. The final temperature is 87.35 °C. What is the specific heat of the substance in cal/g °arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY