
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
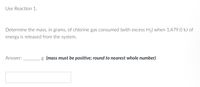
Transcribed Image Text:Use Reaction 1.
Determine the mass, in grams, of chlorine gas consumed (with excess H₂) when 1,479.0 kJ of
energy is released from the system.
Answer:
g (mass must be positive; round to nearest whole number)

Transcribed Image Text:Consider these reactions:
Reaction 1:
H₂(g) + Cl₂(g) 2HCl(g) AH = -184.6 kJ
Reaction 2:
20F2(g)
O₂(g) + 2 F₂ (g) ΔΗ = -49.4 kJ
Reaction 3:
N₂(g) +20₂ (8) 2NO₂(g) ΔΗ =
+66.4 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Use Reaction 2.
How much energy (in kJ) is released when 49.0 g of oxygen difluoride decomposes?
Enter a positive number. Round to the nearest hundredth.
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Use Reaction 2.
How much energy (in kJ) is released when 49.0 g of oxygen difluoride decomposes?
Enter a positive number. Round to the nearest hundredth.
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- || Question 15 A calorimeter holds 25 g water at 25.0°C. A sample of hot iron is added to the water, and the final temperature of the water and iron is 30.0°C. What is the change in en- thalpy associated with the change in the water's temperature? Note: The specific heat of water is 4.18 g°c. Use the formula AH = - cm AT %3D Show your work. IUSarrow_forwardThe following thermochemical equation is for the reaction of carbon monoxide(g) with oxygen(g) to form carbon dioxide(g). 2CO(g) + O2(g)- →2CO2(g) AH=-566 kJ How many grams of CO(g) would have to react to produce 104 kJ of energy? gramsarrow_forwardA chemist measures the energy change AH during the following reaction: Cl₂(g) + H₂(g) → 2 HC1 (g) AH=184. kJ Use the information to answer the following questions. endothermic. This reaction is... 0 exothermic. Suppose 28.0 g of Cl₂ react. Yes, absorbed. Yes, released. Will any heat be released or absorbed? No. If you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed. Round your answer to 3 significant digits. ☐kJ x10 X ?arrow_forward
- Write the equation for the complete combustion of octane. If the heat of combustion of octane is -5294 kJ/mol, determine how many kJ of energy could be obtained from the combustion of one gallon of gasoline (assume the density is 703 kg/m3. Show ALL workarrow_forwardSuppose your body was able to use the chemical energy in gasoline. How far could you pedal a bicycle at 15 km/hr on the energy in 2.2 gal of gas? ( 1 gal of gas has a mass of 3.2 kg.).arrow_forwardWhen salicylic acid, C-H,0,, is burned in a bomb calorimeter with a heat capacity of 2.046 x 10* J/°C, 87.7 kJ heat is produced and the temperature increases to 26.10 °C. Calculate the initial 22 - temperature. O A) 30.39 B) 17.52 C) 15.52 D) 16.00 E) 21.81arrow_forward
- If 585 J of energy are absorbed by 125.6 g of aluminum and the initial temperature is from 20.0 oC, what is the final temperature? Is this endothermic or exothermic?arrow_forwardConsider the following reaction. N2 + 3 H2 -> 2 NH3 ΔH = -92 kJ/mol How many grams of nitrogen gas react if 45 kJ of energy is released? Group of answer choicesarrow_forwardThe heat of combustion for methane (the gas in used for our Bunsen burners) is -890 kJ/mol. Determine how many kJ would be given off when 3.8509 moles of methane is burned.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY