
Physical Chemistry
2nd Edition
ISBN: 9781133958437
Author: Ball, David W. (david Warren), BAER, Tomas
Publisher: Wadsworth Cengage Learning,
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
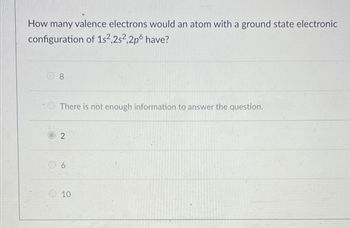
Transcribed Image Text:How many valence electrons would an atom with a ground state electronic
configuration of 1s2,2s2,2p6 have?
8
There is not enough information to answer the question.
2
6
10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The photoelectron spectrum of HBr has two main groups of peaks. The first has ionization energy 11.88 eV. The next peak has ionization energy 15.2 eV, and it is followed by a long progression of peaks with higher ionization energies. Identify the molecular orbitals corresponding to these two groups of peaks.arrow_forwardHere is the ground-state electron configuration of a -1 anion of an unknown Element E. [Kr] 1L1L|1L||1L||1L||1L||1L||1L1L 4d 5 s 5p Use this diagram to answer the questions below. What is Element E? Write its chemical symbol. How many unpaired electron spins are there in this anion?arrow_forward5. The ground state of NO2 is A1. To what excited states may it be excited by electric dipole transitions, and what polarizations of light is it necessary to use to get the transition.arrow_forward
- a.) Based on their ground state electron configurations alone, rank these molecule in order of bond dissociation energy from smallest to greatest:A = N2B = B2C = O2. b.) Based on their ground state electron configurations alone, rank these molecule in order of bond length from smallest to greatest:A = N2B = O2C = NOarrow_forwardFor a Mn3+ ion in its ground electronic state: How many electrons are there in the 4s orbital?arrow_forwardChoose the correct ground state term symbol of high spin octahedral Ir³+ 3F 4F 5D 0³D 9 6Sarrow_forward
- Hello! I need help, please. See picturearrow_forwardPrinciples of Modern Chemistry 8th edition chapter 6 Additional Problem 71arrow_forwardThe ground state of NO2 is A1 in the group C2v. To what excited states may it be excited by electric dipole transitions, and what polarization of light is it necessary to use?arrow_forward
- Give the term symbol(s) correspond to the ground state configuration of a Y2+ ion.arrow_forwardPhotoelectron spectra were acquired from a sample ofgaseous N2 using He(I) light with energy 21.22 eV as theionization source. Photoelectrons were detected withkinetic energy values 5.63 eV and also with 4.53 eV. Calculate the ionization energy for each group of electrons.Identify the MOs that were most likely the sources ofthese two groups of electrons.arrow_forwardGive the ground-state electron configu rations of (a) H2 - . (b) Li2, (c) Be2, (d) C2, (e) N2, and (f) O2 .arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning