
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:A
B 2
C
D
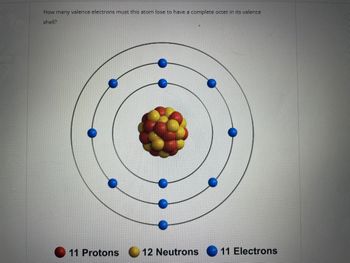
Transcribed Image Text:How many valence electrons must this atom lose to have a complete octet in its valence
shell?
11 Protons
12 Neutrons 11 Electrons
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What charges would the ions need to make the hypothetical ionic compound XY₂? The X ion has a charge of +2, and each Y ion has a charge of -1. The X ion has a charge of +1, and each Y ion has a charge of -2. The charge for the X ion must be +2, whereas the charge of each Y ion is -2. 112. The X ion has a charge of +1, whereas each of the two Y ions has a charge of +arrow_forwardI need the answer as soon as possiblearrow_forwardFill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: Some ionic compounds cation anion empirical formula name of compound 10, K 2+ Cr Bro, K 10,arrow_forward
- o Give the formula for an ionic compound formed from each pair of ions. Na+ and 0²- Mg²+ and S²- Al³+ and F Ca²+ and P³-arrow_forwardFind the ionic compound among these. SO3 O C H4 CaH2 О CаН2 O C2H6arrow_forwardName each ionic compound. In each of these compounds, the metal forms more than one type of ion. Spell out the full name of the compound. HgBr2 Fe2O3 CuI2arrow_forward
- What is the formula of silicon tetrafluoride? SiF4 SiF SiF3 Si₂F4 Si4Farrow_forwardFor each row in the table below, decide whether the pair of elements will form a molecular or ionic compound. If they will, then enter the chemical formula of the compound. If the elements will form more than one compound, enter the compound with the fewest total number of atoms. You may assume all chemical bonds are single bonds, not double or triple bonds. element #1 barium argon carbon element #2 sulfur helium iodine compound formed? chemical formula O ionic O molecular O neither O ionic O molecular O neither O ionic O molecular O neither 0 0 0 00 X 5 ? olo Ararrow_forwardName the following binary ionic compounds, using a Roman numeral to indicate the charge on the metal ion: a CoCl₂: CoCls: b NiS: Ni₂ S3: CMnO: Mn₂ 03: PbF2: PbF4: 10arrow_forward
- For each row in the table below, decide whether the pair of elements will form a molecular or ionic compound. If they will, then enter the chemical formula of the compound. If the elements will form more than one compound, enter the compound with the fewest total number of atoms. You may assume all chemical bonds are single bonds, not double or triple bonds. element #1 hydrogen calcium radon element #2 compound formed? chemical formula O ionic chlorine chlorine neon 100 O molecular Oneither Oionic O O O O O O O molecular O neither O ionic O molecular Oneither 0 90 X 5arrow_forwardProvide the formula for a compound containing 6 chlorine atoms for every 1 sulfur atomarrow_forwardName these ionic compounds: MgS Al2O3 Ca(HSO3)2 Sr3(PO4)2 Fe(OH)2 Pb(CH3COO)2 (NH4)2HPO4arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY