
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
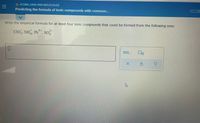
Transcribed Image Text:O ATOMS, IONS AND MOLECULES
Predicting the formula of ionic compounds with common...
Write the empirical formula for at least four ionic compounds that could be formed from the following ions:
Clo, NH, Pb*, so
4+
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the chemical formula and indicate the physical state of the products. Example: PbC12 (s) + H2O (I). carbonic acid (aq) + potassium hydroxide (aq) ==>arrow_forwardA Group 2 metal with an electron configuration of [Ne]3s2 combines with fluorine (F2) to produce a compound. In g/mol, what is the molar mass of the product?arrow_forwardDragonium (Dr) is an element only found in the scales of dragons. It is known to exist only in the oxidation state of +3, and it is a metal. After dragons go for their morning swim in the Pacific Ocean, their scales change color due to the formation of a chlorine salt of dragonium which crystallizes out with four water molecules per each dragonium atom. Select the name of this compound.arrow_forward
- Write a balanced, neutralization reaction for the antacid “Gaviscon” who’s primary active ingredient is aluminum hydroxide, Al(OH)3.arrow_forwardWhat mass of Cu(IO3)2 can be formed from 0.650 g of CuSO4 · 5H2O? What mass of KIO3 is needed to convert the copper in 0.2750 g of CUSO4 - 5H2O to Cu(IO3)2?arrow_forwardThe empirical formula (CH2O) for most carbohydrates indicates that carbohydrates are sweet. this is a complex carbohydrate. a structural formula cannot be drawn from any of these formulae. there is one carbon (C) atom for each water (H2O).arrow_forward
- What is the molar concentration of each ion present in an aqueous solution created by dissolving 23.4 g Na,SO, (MM 142.1 g/mol) in enough water to form 125 mL of solution. %3Darrow_forward10. Write the completed and balanced equation with the correct physical state for each species in molecular, ionic and net ionic form. CS3PO4 (aq) + Ge(C2H3O2)4 (aq) → lonic: Net lonic:arrow_forwardHow do you write the reaction of aqueous copper(ii) ion with the aqueous iodate ion?arrow_forward
- There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. In the first step, calcium carbide and water react to form acetylene and calcium hydroxide: CaC,(s)+2H,O(g)–C,H,(g)+Ca(OH),(s) In the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6 C,H,(g)+3 CO,(9)+4H,0(9)→5 CH,CHCO,H(9) Write the net chemical equation for the production of acrylic acid from calcium carbide, water and carbon dioxide. Be sure your equation is balanced. Explanation Check O 2022 McGraw Hill LLC. AlI Rights Reserved. Terms of Use Privacy Center Accessibility 2:24 PM W 4/8/2022 DELL Esc F1 F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 PrtScr Insert Delete PgUp PgDn Fn C@ 23 2$ Num Lock & Backspace 1 2 3 4 7 8. Co (Oarrow_forwardA chemical characteristic of acids is their ability to react with metals in a chemical reaction to produce what kind of molecular gas? carbon di-oxide gas chlorine gas oxygen gas hydrogen gasarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY