
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:tab
caps lock
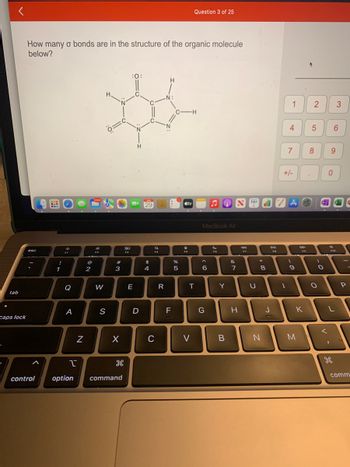
How many o bonds are in the structure of the organic molecule
below?
esc
control
!
1
staps
F1
Q
A
1
N
NO
2
19,842
300
F2
W
0=c
S
#
3
X
H
option command
80
F3
:0:
E
N
H
D
C
C
AIN
29
$
4
a
F4
C
R
H
N:
F
%
d
LO
5
-H
tv
Question 3 of 25
4
FS
T
V
MacBook Air
^
< 6
G
?
FG
Y
B
JONTANAO
&
NK
7
H
97
U
*
8
N
DII
J
1 2
4
1
7
+/-
(
9
DD
K
M
5
8
)
O
D
O
9
0
d
3
6
Q
-
P
comm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- C2H2 a) How many lone pairs (non-bounding electron pairs) does the compound possess on All atoms? (central atom(s) and outer atoms? b) For this compound, Identify the following -number of electron groups (electron domains) -number of atoms bounded to the central atom -number of non-bounding electron pairs (lone pairs) attached to the central atom -General formula c) What is the Electron group Geometry also called "overall shape" and Hybrid Orbital designation for the central atm? d)What is the shape the atoms make (excluding the lone pairs)? This is also called the "molecular shape"arrow_forwardI keep getting these questions wrongarrow_forwardThis is only one question so plz All subparts.arrow_forward
- Draw the Lewis structure for OCS. Answer the following questions for the Lewis structure for OCS , given that carbon is the central atom and all atoms obey the octet rule. 1. How many double bonds exist in this structure? 2. How many electrons surround the carbon atom? 3. How many lone pairs are around the carbon atom? 4. How many lone pairs are around the sulfur atom? 5. How many lone pairs are around the oxygen atom? 6. How many electrons surround the oxygen atom?arrow_forwardHow many constitutional isomers does C2H3Cl3 have? Draw all the isomers using lewis structures.arrow_forwardWhen part of a molecule, what is the typical number of bonds and nonbonding pairs of electrons for each of these atoms? bonds to carbon: nonbonding electron pairs on carbon: bonds to oxygen: nonbonding electron pairs on oxygen: bonds to sulfur: nonbonding electron pairs on sulfur: bonds to halogens (F, Cl, Br, I): nonbonding electron pairs on halogens: bonds to nitrogen: nonbonding electron pairs on nitrogen: bonds to phosphorus: nonbonding electron pairs on phosphorus: bonds to hydrogen: nonbonding electron pairs on hydrogen:arrow_forward
- Draw Lewis structures for the following compounds. a) CH2Oarrow_forwardHow many pairs ( set or two electrons together ) are in the Lewis for structure of carbon ?arrow_forwardStructural Isomers: In the next part you will learn a little about structural isomers and how to draw them. Structural isomers are compounds with the same molecular formula, but different bonding arrangements or connectivities. In other words, the atoms are connected in a different order. For example, there are 2 different compounds with the molecular formula C4H10. They are given below: H H-C- H -H H H HHH H- H H-C -H Η ΗΗ H H Do you see how the atoms are bonded in a different order in the 2 structures? So they are structural isomers. C3H12 Find three different molecular structures, isomers, with this formula. (Hint: Remember the C rule where every C atom needs to have 4 bonds.) C2H60 Find two different molecules with this formula. (Hint: What are the 2 ways you can C5H10 How many structural isomers can you find for this formula? (Hint: Notice that 2 H atoms have been removed. This means that there is EITHER 1 double bond OR there is a ring structure.) Try to provide at least 4…arrow_forward
- Jj.11.arrow_forwardIndicate the number of single, double, and triple bonds in each of the three compounds. СH,O has single bonds, double bonds, and triple bonds. C,H, has single bonds, double bonds, and triple bonds. Со, has single bonds, double bonds, and triple bonds. careers prvacy po ig tems of use contact us belparrow_forwardMolecular Formula SO₂ SiCl4 Total # of valence electrons CH₂Cl₂ Lewis Structure # of Electron Groups Bond Electron Geometry Angle Molecular Geometry QUESTION 1. Using the molecular model kit, make and compare the molecular models for CO₂, H₂O, and SO₂. Even though each molecule has 3 atoms, the molecular geometries (shapes) are very different. Explain why the shapes are different. on. -C Polar or Nonpolar Attractive Force O <arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY