
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:101 Chem101
C How Many Moles Of HCIN X
Answered: How many mo X
Construct the expression X
My Questions | bartleby
+
app.101edu.co
S O
2d
UC mail
Canvas
I UC microsoft
108" Wide Quilt Bac...
14 Google Calendar-.
101 Chem101
b My Solutions | bartl..
Google Docs
Question 44 of 52
Submit
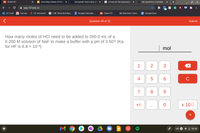
How many moles of HCl need to be added to 200.0 mL of a
0.200 M solution of NaF to make a buffer with a pH of 3.50? (Ka
for HF is 6.8 × 10-4)
| mol
3
4
6.
C
7
8.
9.
+/-
х 100
M 9
US
18:26
...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the pH of a buffer made by combining 45.0 mL of 0.150 M nitrous acid and 20.0 mL of 0.175 M sodium nitrite? The Ka of nitrous acid, HNO2, is 4.5×10–4.arrow_forwardA 1.0-L buffer solution contains 0.100 mol acid, HA, and 0.100 mol base, NaA. The value of K, for the acid HA is 1.8x104. Ignoring the small volume change, what is the most likely pH of the solution if a 10.0 mL- sample of 1.00 M HCI is added to the original buffer? O 5.50 < pH < 5.74 O 3.51 < pH < 3.69 O 4.10 < pH < 4.22 O 1.20 < pH < 1.45arrow_forwardA 80.00 mL solution initially contains 0.300 M HCN and 0.200 M KCN. What is the new pH after 1.0 mL of 2.5 M HCl is added? Ka = 4.9 x 10–10arrow_forward
- For 480.0 mL of a buffer solution that is 0.120 M in HC2H3O2 and 7.50×10−2 M in NaC2H3O2 , calculate the initial pH and the final pH after adding 1.5×10−2 mol of HCl . ( Ka(HC2H3O2)=1.8×10−5 .)arrow_forwardWhat is the pH of a buffer solution that is 0.214 M in ammonia and 0.390 M in ammonium chloride (NH4CI)? The Kü of ammonia is 1.8 x 10-5. (Two decimal places)arrow_forwardConsider a buffer made from 255 mL 0.10M HC2H:O2 and 125 mL 0.10 NaC2H:O2. Ka for HC2H3O2 is 1.8 x 105. Choose the true statement. A) Adding 25 mg NaOH (s) will reduce the pH. B) Adding 25 mL water will increase the pH. C) Adding 25 mL 0.0050 M HCI will increase the pH. D) Adding 25 mL 0.0050 M HF will reduce the pH. E) Adding 25 mg NaCl (s) will increase the pH.arrow_forward
- A buffer is made by adding 19.4 grams of lithium fluoride to 200.0 mL of 2.00M HF. a) What is the pH of this system before any strong acid or base is added? b) What is the pH after the addition of 200.0 mL of 0.500M HCl? c)What is the pH after the addition of 400.0 mL of 0.500M HCl?arrow_forwardA 1.0 liter solution contains 0.745 M hydrogen fluoride (HF) and 0.786 M sodium fluoride (NaF). What is the pH of this solution? (Ka for HF is 7.2 x 10 –4) How many moles of solid sodium acetate would have to be added to 2.0 L of 0.293 M acetic acid solution to achieve a buffer of pH 4.77? Assume there is no volume change. Ka for HC2H3O2 is 1.8 × 10 –5 What molar ratio of sodium acetate to acetic acid, is required to create a buffer solution having a pH of 4.58 at 25°C? Ka for HC2H3O2 is 1.8 × 10 –5.arrow_forwardMoly Cule is titrating 0.2 M of a strong base (NaOH) into 100 ml of a solution of 0.1M weak acid (HAc). Ka for HAc is1.8x10 -5. How much NaOH must be added to reach the equivalence point, and what is the pH at the equivalence point?arrow_forward
- Let’s build a buffer using hypobromous acid (HBrO, Ka = 2.3×10−9) and hypobromite ion (BrO−). If [HBrO] is 0.100 M, then what concentration of hypobromite ion is needed to achieve pH = 8.50arrow_forwardA buffer solution is 0.462 M in HCN and 0.283 M in KCN. If Ką for HCN is 4.0 × 10-¹0, what is the pH of this buffer solution? pH =arrow_forwarda) Calculate the pH of a solution containing 0.30 M HF and 0.30 M NaF.The Ka for HF is 7.2×10-4. pH = b) Carbonate buffers are important in regulating the pH of blood at 7.40. If the carbonic acid concentration in a sample of blood is 0.0014 M, determine the bicarbonate ion concentration required to buffer the pH of blood at pH = 7.40. Ka = 4.3×10-7 Сoncentration = M c) You have 75.0 mL of 0.10 M HA. After adding 30.0 mL of 0.10 M NaOH , the pH is 5.50. What is the Ka value of HA ? = d) Consider the titration of 80.0 mL of 0.100 M Ba(OH)2 by 0.400 M HCl. Calculate the pH of the resulting solution after the following volumes of HCl have been added. 40.0 mL pH = 50.0 mL pH =arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY