
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
a) From the chemical structure above, write the amino acid sequence using both three-letter and one-letter abbreviations. Disregard chirality for this question.
b) Indicate adjacent to the alpha-carbon of each residue whether it has an L- or D-configuration.
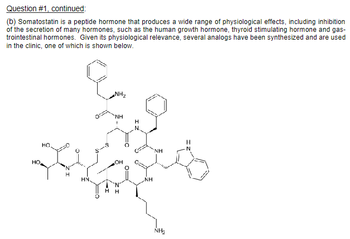
Transcribed Image Text:Question #1, continued:
(b) Somatostatin is a peptide hormone that produces a wide range of physiological effects, including inhibition
of the secretion of many hormones, such as the human growth hormone, thyroid stimulating hormone and gas-
trointestinal hormones. Given its physiological relevance, several analogs have been synthesized and are used
in the clinic, one of which is shown below.
HO
HO.
H
HN
NH₂
NH
OH
H
N.
NH
NH
ŃH₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
How do you identify the N vs C terminals? For the first part of the question, do you then list the amino acids in order clockwise from N terminal to C terminal?
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
How do you identify the N vs C terminals? For the first part of the question, do you then list the amino acids in order clockwise from N terminal to C terminal?
Solution
by Bartleby Expert
Knowledge Booster
Similar questions
- Among these amino acid combinations listed above, only the combination of Lys and Glu have side chains with groups that have the greatest ability to stabilize the tertiary structure of a protein. Explain by drawing (a) why Lys and Glu side chain interaction stabilizes the tertiary structure of a protein (b) why the pairs of Glu and Asp & Arg and Pro cannot provide the stability to the protein structure.arrow_forwardThe bottom peptide segment is found in the secondary structure composed of anti-parallel - sheets. From the C-terminus of the peptide below, the sequence immediately after alternation contains the following amino acid residues (from N → C) A-F-R-M-Y-A. a) which side do you expect to face the solvent? Briefly explain your reasons NH, но Nterminusarrow_forwardIdentify the 20 amino acids and their corresponding three-letter and one-letter abbreviationsarrow_forward
- с" CH2 CH2 "НaN H с. CH С" CH Нзс CHз ZI о-оarrow_forwardConsider a short peptide with the sequence M-C-Q-L-Y-P-E-D-K. List the ionizable groups in this peptide, and the net charge of the whole peptide at pH a) 1.5, b) 7.0, and c)12.0.arrow_forwardThere is parts A-D for the picture provided. A) A peptide has the sequence, Arg-Cys-His-Tyr-Glu-Asn-Lys-Asp. What is the net charge at pH 1? Choices: -5 -4 -3 -2 -1 0 +1 +2 +3 +4 +5 B) A peptide has the sequence Arg-Cys-His-Tyr-Glu-Asn-Lys-Asp. What is the net charge at pH 5? Choices: -5 -4 -3 -2 -1 0 +1 +2 +3 +4 +5 C) Same Peptide sequence, What is the net charge at pH8.5, (N-terminus is pronated) Choices: -5 -4 -3 -2 -1 0 +1 +2 +3 +4 +5 D) A peptide has the sequence, Arg-Cys-His-Tyr-Glu-Asn-Lys-Asp. What is the net charge at pH 13? Choices: -5 -4 -3 -2 -1 0 +1 +2 +3 +4 +5 Thank You!arrow_forward
- Proline is one of the amino acids in the "special" groups of sidechains. One reason for this is the dramatic impact it can have on secondary structure. a) draw the lewis structure for a tripeptide with the sequence Ala-Pro-Ala b) Proline residues are rarely found in alpha helices - in fact, they are often referred to as helix-disrupting amino acids. Thinking about intermolecular interactions, provide an explanation for why proline might disrupt alpha-helices. c) Proline also has a similar effect on beta-sheets, and is rarely found in the middle of beta sheets. Thinking about noncovalent interactions, provide an explanation for why proline might disrupt beta-sheets.arrow_forwardConsider an amino acid (A) with no ionizable side chains, and call the three species involved in the acid/base equilibria H2A+, HA, and A- (see scheme below). Assume that pKa(1) = 2.0 and that pKa(2) = 9.0. Suppose that the total concentration of the amino acid is 1.0 mM. Report to two significant digits. pH [H2A+] (mM) [HA] (mM) [A-] (mM) 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 10.0 11.0 12.0arrow_forwardcan u help mearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON