
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
How do you find the standard deviation and average deviation using the data table above?
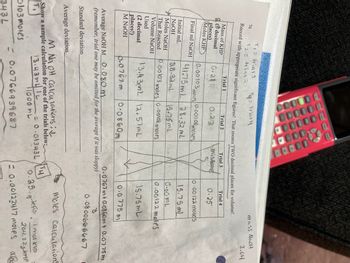
Transcribed Image Text:T₁ = trig11
Tq: Trialy
T2 = trial 2
Record with appropriate significant figures! That means TWO decimal places for volume!
Trial 1
Trial 2
Trial 4
0.25
76
Mass of KHP
decimal
places)
Moles KHP
Final ml NaOH
Initial mL
NaOH
Moles NaOH
That Reacted
Volume NaOH
Used
(2 decimal
places!)
M NaOH
0.29
Dlo3 moles
343L
0.22
0.00103 moles
0.00108 moles
41.75mL
28.32 ml
28.32mL
15.75ml
0.00103 moles 0.00108 moles
13.43m2 12.57mL
0.0860m
Trial 3
mistake
0.0767 m
Average NaOH M 0.080 m
(remember, trial one may be omitted for the average if it was sloppy)
Standard deviation
Average deviation
M Na CH Calculations: 1
Show a sample calculation for one of the trials below:
13.43m4
IL
= 0.01343L
11000 ML
- 0.0766939687
mass Naot
0.00 122 moles
ML C.00 ML
15.75mL
0.00122 males
15.75mL
0.0 775 m
2.04
0.0767m+ 0.0860m + 0.0775m
0.0800666667
Moles Calculations
TH
0.25 KP.
I mol KHP
204.22kMP
= 0.00122417 moles aba
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How did you get 0.32?Also where did you get 500 for Vmax? In the question it says 50arrow_forwardThree trials for this experiment had been conducted and the following are the masses of limiting reactant: 0.285 grams, 0.299 grams, and 0.292 grams. What is the standard deviation for the masses of limiting reactant for the three trials? please show work on how to do.arrow_forward2. A student performs an experiment Her data is below: Table 1. Masses Before and After Pulling Steel Wool Apart Trial 1 Trial 2 Trial 3 158.93 g 157.45 g 145.67 g Initial mass of steel wool Final mass of steel wool Difference AVERAGE 158.92 g -0.01 g 157.48 g 0.03 g -0.003 g 145.64 g -0.03 g Draw a diagram to show the particles before and after pulling: Before Afterarrow_forward
- a. Assuming that the expected volume (true value) for the 10 mL graduated cylinder is exactly equal to the line you used for the calibration (i.e. 10.0000 mL if you used the 10 mL line), what is the percent error for your volume?arrow_forwardI measured a line that was 8.4 centimeters which is the same as 3.375 inches. How do I calculate the percente relative error?arrow_forwardAnswer both questions with details solution I'll give you ?arrow_forward
- Find the volume and density of an irregular piece of the mineral quartz is found to weigh 12.4g. It is then placed into a graduated cylinder that contains some water. The quartz does not float. The water in the cylinder was at a level of 25.2ml before the quartz was added and at 29.9ml afterward.arrow_forwardPlease help with question 7arrow_forwardI need help with how many miles there must have been in the sample?arrow_forward
- Correct! Now, determine the uncertainty value for the measurement. 7.48 ± mL 7 SUBMIT UNCERTAINTY 00arrow_forwardI need help wilth calculating percent relative error. What will be the real value when it comes to length and centimeters?arrow_forwardDetermine which type of sampling technique is used. 19. A bread company wants to know whether men or women prefer their brand of bread so they randomly select from a group of men and a randomly select from a group of women for the sample. 20. Before leaving the room, the teacher asks students to write the number of hours they studied for the test on the chalkboard if they want to participate. 21. An electronics company wants to know which feature on one of their electronic devices is their customers' favorite. All customers who call technical support are surveyed. Choices for 19-28: A) Simple random sample B) Stratified sample C) Systematic sample D) Convenience sample E) Voluntary response sample 22. A corporation wants to know if its employees would rather work more days with less hours per week or less days with more hours per week. They randomly choose employees for the survey. 23. Brianna wants to figure out college students' thoughts about the availability of parking spaces on…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY