
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Can someone help me solve this? Thank u!
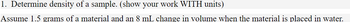
Transcribed Image Text:1. Determine the density of a sample. (show your work WITH units)
Assume 1.5 grams of a material and an 8 mL change in volume when the material is placed in water.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Why is it in Cm3 and not mL?
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Why is it in Cm3 and not mL?
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3arrow_forward66 Match the description with the name of the drug. [Sorry there is no partial credit for matching questions) what oplate is found in some cough syrups? What drug is used to treat heroin overdosing? What drug is used to treat heroin addiction, but it is still addictive What drug is used in a patch for chronic pain management? Tolerance for a drug means that over time the dosage can be decreased to have the same effect as before. True False Opiates are all addictive True False Methadone answer not given novocaine "Narcan" or naloxone codeine fentanyl Endorphins and morphine bind to the same receptor sites in the brain True False Acupuncture causes the body to produce endorphins which is the body's own pain killers True False The histamine molecule that sits on the receptor sites in the respiratory system is a different molecule than the one that affects the receptor sites in the stomach True Falsearrow_forwardQuestions 15 through 20 pertain to the following medication formula: Here is the formula a pain and inflammation reducing ointment. Methyl salicylate (sp gr = 1.167) 60 mL Lidocaine 4% (w/w) Tween:Span mix (1:4 mix) Polypeg Base 10 g QS to 0.5 kg You receive an order to make 3 solid ounces of this product. Answer the following questions pertaining to producing 3 solid ounces of the product. 15. Volume of methyl salicylate, in milliliters. 16. Weight of lidocaine in grams. 17. Weight of Tween in milligrams. 18. Weight of Span, in milligrams. 19. Weight of Polypeg base, in grams. 20. What is the final concentration of methyl salicylate, as a percent concentration (w/w)?arrow_forward
- Unit 3-Two slides 11. Create a question that involves your compound and the formula m= n.M. Solve the question and show all of your work. wwwww 12. Create a question that involves your compound and the formula n = N/NA. Solve the question and show all of your work. TEarrow_forwardIn an alternate reality, the following salinity vs. density information was collected and summarized below. Based on this data, calculate the salinity of a solution that has a density of 1.017 g/mL. Enter your answer with at least 3 significant figures. Salinity (g/mL) Density (g/mL) 0.00 0.983 20.00 0.998 50.00 1.020 100.00 1.057 150.00 1.095arrow_forwardA protocol calls for 1 liter of solution that is 1.5 % (w/v) NaCl, 5mM Tris (pH 8.0), 10% (w/v) Carnation Instant Milk (we really do use this in lab!), and 0.03% (v/v) Tween-20 detergent. How do you make this solution?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY