
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
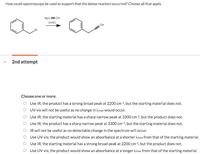
Transcribed Image Text:How could spectroscopy be used to support that the below reaction occurred? Choose all that apply.
Nac= CH
DMSO
CH
2nd attempt
Choose one or more:
O Use IR; the product has a strong broad peak at 2200 cm-1, but the starting material does not.
O UV-vis will not be useful as no change in Amax Would occur.
O Use IR; the starting material has a sharp narrow peak at 3300 cm-1, but the product does not.
O Use IR; the product has a sharp narrow peak at 3300 cm-1, but the starting material does not.
IR will not be useful as no detectable change in the spectrum will occur.
Use UV-vis; the product would show an absorbance at a shorter Amax from that of the starting material.
O Use IR; the starting material has a strong broad peak at 2200 cm-1, but the product does not.
Use UV-vis; the product would show an absorbance at a longer Amax from that of the starting material.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- PLS ANSWER ONLY THE CIRCLED ONE! I just need the answer. Not a long paragraph. Just answer.arrow_forwardurgent :i am supposed to match the peaks on the compound to where it is on the nmr spectrscopy. i started matching but am not sure if i am right, please help finish and correct if wrongarrow_forwardWhat type of compound is most consistent with the IR spectrum shown below? LOD 5D D 4000 3000 aldehyde alkene None of the choices are correct. carboxylic acid ester nitrile (i.e., RCN) O ketone 2000 1500 m что p 1000 500arrow_forward
- Can you please explain the stepsarrow_forwardhelp me please. thanksarrow_forwardAssigning Spectra: The peak at 0.9 ppm is a doublet, integrating to 6. Assign this peak to a fragment (Which statement is most correct?) * 20 10 ppm R2-CH-CH2-C(=0)-OR. Based on shift it could be a CH2 near carbonyl, with one neighboring proton. R2-CH-CH3. Could be a standard methyl, with one neighboring proton. O R-CH2-CH3. Could be a standard R-CH3, with 2 neighboring protons. RO-CH(R)-CH3. Could be standard methyl, with beta substituent, and one neighboring proton.arrow_forward
- Please help me pleasearrow_forwardA compound and its ¹H-NMR spectrum are shown below. Assign peak 1 and peak 2 in the spectrum to the hydrogen(s) labeled in red. i. a H3C- 10 C4H8O₂ Compound C b c -O—CH,CH3 2006 Brooks/Cole Thomson Submit Answer C 9 ↑ Which labeled hydrogen(s) correspond to each peak? peak 1: peak 2: 8 7 6 2H 5 Chemical Shift (8) Peak 3 Retry Entire Group 6 more group attempts remaining 3. 3H Peak 2 3H Peak 1 0 ppmarrow_forward5. The proxluct of the reaction below gives the IR spectrum shown. Although you are unfamiliar with the rcaction, usc your knowledge of IR spectroscopy to predict a likely product. (Note: the number of carbon atoms in the product is the same as in the starting material.) Page 4 OH Al(OiPr)3 acetone no 4000 c hle belveen sall rlate cas. e Ged LIFEarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY