
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
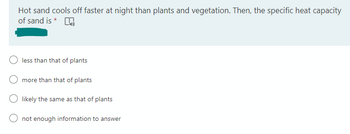
Transcribed Image Text:Hot sand cools off faster at night than plants and vegetation. Then, the specific heat capacity
of sand is *
less than that of plants
more than that of plants
likely the same as that of plants
not enough information to answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 4) A ground source heat pump heats a building by extracting heat from the ground and pumping it into the building. Define: Qc = heat extracted from the ground, Qn= heat pumped into the building, W = electric energy used by the heat pump, Tc= temperature of the ground, T = temperature of building (Qc, Qn, and W are positive by definition). Assume Tr > Tc. a) Draw a diagram showing energy flow in and out of the heat pump. b) Write a general expression for the change AS in the entropy of the "universe", that is the heat pump plus the cold and hot reservoirs, in terms of the quantities defined above. Now assume ideal (reversible) operation, and take Te= 10 °C and Tp= 20 °C. What is the coefficient of performance (ČOP) of the heat pump? By what factor would this change if we had Tc = 0 °C instead?arrow_forwardThe urban heat island effect is often strongest at night when temperatures would typically drop. What does this indicate about energy transfer and the material used in the building? (a) The material in buildings releases more energy at night than it absorbs during the day (b) The material in buildings absorbs a lot of energy during the day and when it gets colder at night the energy is released back into the cooler air. (c) The material in buildings absorbs energy during the day and releases it because the temperature of the buildings is lower than the cooler air (d) The material in buildings releases a lot of energy into the hot air during the day so at night all of the energy is still therearrow_forward#1. What are the three mechanisms for heat transfer and how do they differ?arrow_forward
- In the table below for E106, which of the following is the amount of heat lost by the metal specimen when dropped in the calorimeter with water? TRIAL 1 Determining the Specific Heat of Metal Specimen Metal Specimen 1 Mass of calorimeter, me Mass of calorimeter with water Mass of water, mi Mass of metal specimen, mm Initial temperature of metal, tom Initial temperature of calorimeter, toc Initial temperature of water, tow Final temperature of the mixture, tmix Specific Heat of Water cw Specific Heat of calorimeter ce Experimental specific heat of metal, cm Actual specific heat of metal, cm Percentage Error O 699 cal O 212 cal O 675 cal O 488 cal 150.0 g 225.0 g 8 100.0 g 100°C 20.0°C 20.0°C 26.5°C 1.0000 çal/gC 0.2174 cal/gC cal/gC 0.0917 cal/gC %arrow_forwardA block has a temperature of 277 K. Heat could spontaneously flow from which of the following objects to the block? Choose all that apply. a) A counter top at 357 K b) A carpet at 177 K c) A rug at 324 K d) A wall at 246 K e) A brick at 361 K f) A board at 106 K g) A table 366 K h) A shelf at 277 Karrow_forwardExample: Ice-cold Lemonade Ice at 0°C is placed in a Styrofoam cup containing 0.32 kg of lemonade at 27°C. The specific heat capacity of lemonade is virtually the same as that of water. After the ice and lemonade reach and equilibrium temperature, some ice still remains. Assume that mass of the cup is so small that it absorbs a negligible amount of heat. (@b_² = SMAD)+¹=² Heat lost by lemonade Heat gained by icearrow_forward
- SKIN temperatures (57 C)the iatent neat or vap moi water ny of watch p=998.2 kg/m'. 3. Assume that the thickness of the tissue between the interior and the exterior of the body is d =3 cm and that the average area through which conduction can occur is A =1.5 m². Find heat transferred by conduction per hour if temperature difference between the inner body and the skin is AT = 2°C. Coefficient of thermal for tissue without blood is K. = 18 × 10-2 Cal /m-hr-ºC.arrow_forwardSoil heats up much faster than water when the two are exposed to sunlight. Use that fact and your understanding of heat transfer to predict which way the wind will blow near the surface of the earth as the sun rises near the seashore. Explain.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON