
University Physics Volume 2
18th Edition
ISBN: 9781938168161
Author: OpenStax
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
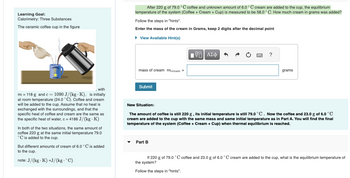
Transcribed Image Text:Learning Goal:
Calorimetry: Three Substances
The ceramic coffee cup in the figure
with
m = 118 g and c = 1090 J/(kg K), is initially
at room temperature (24.0 °C). Coffee and cream
will be added to the cup. Assume that no heat is
exchanged with the surroundings, and that the
specific heat of coffee and cream are the same as
the specific heat of water, c = 4186 J/(kg.K)
In both of the two situations, the same amount of
coffee 220 g at the same initial temperature 79.0
C is added to the cup.
But different amounts of cream of 6.0 °C is added
to the cup.
note: J/(kg K) =J/(kg. °C)
After 220 g of 79.0°C coffee and unknown amount of 6.0 °C cream are added to the cup, the equilibrium
temperature of the system (Coffee + Cream + Cup) is measured to be 58.0 °C. How much cream in grams was added?
Follow the steps in "hints".
Enter the mass of the cream in Grams, keep 2 digits after the decimal point
► View Available Hint(s)
mass of cream mcream =
Submit
15| ΑΣΦ
Part B
www
?
grams
New Situation:
The amount of coffee is still 220 g, its initial temperature is still 79.0 °C. Now the coffee and 23.0 g of 6.0 °C
cream are added to the cup with the same mass and same initial temperature as in Part A. You will find the final
temperature of the system (Coffee + Cream + Cup) when thermal equilibrium is reached.
If 220 g of 79.0 °C coffee and 23.0 g of 6.0 °C cream are added to the cup, what is the equilibrium temperature of
the system?
Follow the steps in "hints".
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- At 25.0 m below the surface of the sea, where the temperature is 5.00C, a diver exhales an air bubble having a volume of 1.00 cm3. If the surface temperature of the sea is 20.0C, what is the volume of the bubble just before it breaks the surface?arrow_forwardFor the human body, what is the rate of heat transfer by conduction through the body's tissue with the following conditions: the tissue thickness is 3.00 cm, the difference in temperature is 2.00 , and the skin area is 1.50 m2. How does this compare with the average heat transfer rate to the body resulting from an energy intake of about 2400 kcal per day? (No exercise is included.)arrow_forwardThe specific heat of substance A is greater than that of substance B. Both A and B are at the same initial temperature when equal amounts of energy are added to them. Assuming no melting or vaporization occurs, which of the following can be concluded about the final temperature TA of substance A and the final temperature TB of substance B? (a) TA TB (b) TA TB (c) TA = TB (d) More information is needed.arrow_forward
- Compare the rate of heat conduction through a 13.0-cm-thick wall that has an area of 10.0 m2 and a thermal conductivity that of glass wool with the rate of heat conduction through a 0.750-cm-thick window that has an area of 2.00 m2, assuming the same temperature difference across each.arrow_forwardAt high noon, the Sun delivers 1 000 W to each square meter of a blacktop road. If the hot asphalt transfers energy only by radiation, what is its steady-state temperature?arrow_forwardAn astronaut performing an extra-vehicular activity (space walk) shaded from the Sun is wearing a spacesuit that can be approximated as perfectly white ( e=0 ) except for a 5 cm × 8 cm patch in the form of the astronaut's national flag. The patch has emissivity 0.300. The spacesuit under the patch is 0.500 cm thick, with a thermal conductivity k 0.0600 W/m , and its inner surface is at a temperature of 20.0 . What is the temperature of the patch, and what is the rate of heat loss through it? Assume the patch is so thin that its outer surface is at the same temperature as the outer surface of the spacesuit under it. Also assume the temperature of outer space is 0 K. You will get an equation that is very hand to solve in closed form, so can solve it numerically with a graphing calculator, with software, or even by trial and error with a calculator.arrow_forward
- Some gun fanciers make their own bullets, which involves melting lead and casting it into lead slugs. How much heat transfer is needed to raise the temperature and melt 0.500 kg of lead, stating from 25.0 ?arrow_forwardA 100-g piece of copper, initially at 95.0C, is dropped into 200 g of water contained in a 280-g aluminum can; the water and can are initially at 15.0C. What is the final temperature of the system? (Specific heats of copper and aluminum are 0.092 and 0.215 cal/g C. respectively.) (a) 16C (b) 18C (c) 24C (d) 26C (e) none of those answersarrow_forward(a) If the partial pressure of water vapor is 8.05 torr, what is the dew point? (760 torr = I atm 101, 325 Pa) (b) On a warn day when the air temperature is 35 and the dew point is 25 , what are the partial of the water in the air and the relative humidity?arrow_forward
- A temperature of 162F is equivalent to what temperature in kelvins? (a) 373 K (b) 288 K (c) 345 K (d) 201 K (e) 308 Karrow_forwardWhat would be the final temperature of the pan and water in Calculating the Final Temperature When Heat Is Transferred Between Two Bodies: Pouring Cold Water in a Hat Pan if 0.260 kg of water was placed in the pan and 0.0100 kg of the water evaporated immediately, leaving the remainder to come to a common temperature with the pan?arrow_forwardRubbing your hands together warms them by converting work into thermal energy. If a woman rubs her hands back and forth for a total of 20 rubs, at a distance of 7.50 cm per nub, and with an average frictional force of 40.0 N, what is the temperature increase? The mass of tissues warned is only 0.100 kg, mostly in the palms and fingers.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning