
World of Chemistry, 3rd edition
3rd Edition
ISBN: 9781133109655
Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher: Brooks / Cole / Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
What is the synthesis with aldehyde , ketone using a reagent?
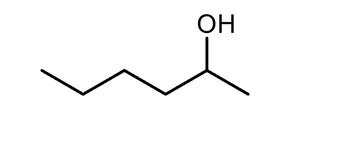
Transcribed Image Text:HO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- HO HO.arrow_forwardIdentify the selected functional groups. Type the name of the functional groups In the boxes Da CH3 HN- CH3 HN O HO NH2, OHarrow_forward* Intranet - CSUM 101 Chem101 O Post Attendee - Zoom b My Questions | bartleby A app.101edu.co Bders Question 4 of 5 Submit advuuned wo,wth yor ameo e Leturelow in 20min teratof it If molecules of hydrogen, nitrogen, oxygen and chlorine have the same kinetic energy which molecule will be moving the fastest? A) hydrogen B) nitrogen C) охудen D) chlorine E) All molecules will have the same speed. 8:13 AM O Type here to search 11 10/20/2020 ...arrow_forward
- PREDICT THE MADOR OR NO RACTION Ot 2 KMNOy, oH, HE ATarrow_forwardO Course Home b Chemistry Question | bartleby G indivuials with austium good to -> A openvellum.ecollege.com/course.html?courseld=16561285&OpenVellumHMAC=446c877376a9bc7ac878d8ad119f4717#10001 Q * E Apps G Gmail O Maps E Connect - To Do As... с ОССС Мoodle P chem work b help Balance Chemical E. YouTube Part A Classify the following molecule according to its functional group. H H H | H-C-C-N-C-C-H | | | | H H H H Enter the name of the class of organic compound. • View Available Hint(s) This molecule is classified as a(n) Submit Part B Classify the following molecule according to its functional group. CH3CH2CH2COOH The molecule contains a(n) functional group. Submit Request Answer 11:01 PM O Type here to search 99+ 3/2/2021 I-Zarrow_forwardO Course Home b Chemistry Question | bartleby G indivuials with austium good to -> A openvellum.ecollege.com/course.html?courseld=16561285&OpenVellumHMAC=446c877376a9bc7ac878d8ad119f4717#10001 Q * E Apps O Maps E Connect - To Do As... O OCCC Moodle P chem work b help Gmail YouTube Balance Chemical E. II Review | Constants | Periodic Table A carbon atom is chiral if it is bonded to four different groups. For example, CHCIBII is chiral, but CCl,BrI is achiral because some of the bonded groups are the same. If a chiral carbon atom is present, then that molecule has a non-superimposable mirror image called an enantiomer. (Figure 1) Part A Identify all the chiral atoms in the below structure by right-clicking* a chiral atom to bring up a menu that includes "Atom Properties." Click on Atom Properties then click the checked box next to the Map field to clear the checkmark. Then enter "1" in the Map *Mac users: Use an equivalent for right- field box to label that chiral carbon atom. clicking.…arrow_forward
- For each compound below, draw in all missing lone pairs and indicate whether each lone pair is delocalized or localized. Then, use that information to determine the hybridization state for each atom that has a lone pair. Hint: allylic lone pairs are delocalized and do not count towards the steric number.arrow_forward13arrow_forwardNOZ ?. pdarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
