
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Ll.125.
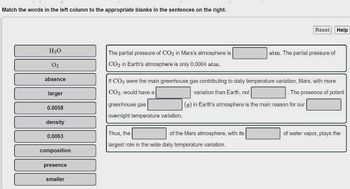
Transcribed Image Text:Match the words in the left column to the appropriate blanks in the sentences on the right.
H₂O
03
absence
larger
0.0058
density
0.0063
composition
presence
smaller
The partial pressure of CO₂ in Mars's atmosphere is
CO₂ in Earth's atmosphere is only 0.0004 atm.
greenhouse gas
overnight temperature variation.
If CO₂ were the main greenhouse gas contributing to daily temperature variation, Mars, with more
CO₂, would have a
variation than Earth, not
. The presence of potent
(g) in Earth's atmosphere is the main reason for our
Thus, the
largest role in the wide daily temperature variation.
of the Mars atmosphere, with its
Reset Help
atm. The partial pressure of
of water vapor, plays the
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment Lavoisier found that mercury(II) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury(II) oxide (HgO) into liquid mercury and gaseous dioxygen. 2HgO + heat 2Hg (1) + O2(g) 2. Suppose 40.0 mL of dioxygen gas are produced by this reaction, at a temperature of 110.0 °C and pressure of exactly 1 atm. Calculate the mass of mercury(II) oxide that must have reacted. Be sure your answer has the correct number of significant digits. X x10 A 18 Ar 8.arrow_forwardpackets of the anhydrous form of a hydrate are sometimes used to keep cellars from being damp. Is there a limit to how long a packet could be used?arrow_forwardThe great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment Lavoisier found that mercury(II) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury(II) oxide (HgO) into liquid mercury and gaseous dioxygen. х10 2. Suppose 14.0 mL dioxygen gas are produced by reaction, at a temperature of 110.0 °C and pressure of exactly 1 atm. Calculate the mass of mercury(II) oxide that must have reacted. Be sure your answer has the correct number of significant digits.arrow_forward
- Determine the concentrations of hydronium and hydroxide ions in 0.076733152673 M aqueous sodium hydroxide.Kw = 1.0E-14. a. Hydroxide ion concentration? b. Hydronium ion concentration?arrow_forwardThe great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment Lavoisier found that mercury(II) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury(II) oxide (HgO) into liquid mercury and gaseous dioxygen. 2. Suppose 53.0mL of dioxygen gas are produced by this reaction, at a temperature of 50.0°C and pressure of exactly 1atm. Calculate the mass of mercury(II) oxide that must have reacted. Be sure your answer has the correct number of significant digits.arrow_forward1. Which of the following is not a step in preparing a water sample container?a. All sample containers must be dark in colorb. The type of sample container and the level of cleaning required depend on the type of sample to be takenc. All sample containers must be thoroughly cleaned in the laboratory before sampling is carried outd. The number of containers prepared must always be in excess of what is needed, for quality assurance, quality control and reserves 2. The purpose of environmental sample analysis is..a. To determine the origin and concentration of chemicals in the environmentb. To determine the origin, concentration of chemicals and/or pollutants in the environmentc. To determine the concentration of a chemical in the environmentd. To determine the cause and concentration of pollutants in the environmentarrow_forward
- For eac te, click the button under the better solvent. solute Which is the better solvent? :0: || :0: :0: CH,-S CH, НО -С — СH, — CH, — С — ОН CH;CH,OH CH; H C Harrow_forwardAşağıda verilen reaksiyonları tamamlayınız.arrow_forwardThe great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment Lavoisier found that mercury(II) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury(II) oxide (HgO) into liquid mercury and gaseous dioxygen. 0 2. Suppose 62.0 mL of dioxygen gas are produced by this reaction, at temperature of 130.0 °C and pressure of exactly 1 atm. Calculate the mass of mercury(II) oxide that must have reacted. Round your answer to 3 significant digits. g 010 X 0x12arrow_forward
- The great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment Lavoisier found that mercury(II) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury(II) oxide (HgO) into liquid mercury and gaseous dioxygen. 2. Suppose 71.0 mL of dioxygen gas are produced by this reaction, at a temperature of 50.0 °C and pressure of exactly 1 atm. Calculate the mass of mercury(II) oxide that must have reacted. Be sure your answer has the correct number of significant digits.arrow_forwardEtO NaOEt, ELOH EtO,C- OEt a) b.) CI EtO HO ČI ÓEt ÓH d.) e)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY