
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
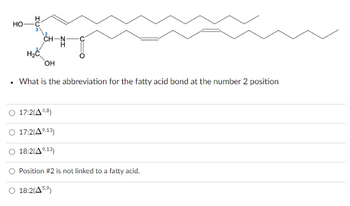
Transcribed Image Text:HO
.
-N-H₂
OH
What is the abbreviation for the fatty acid bond at the number 2 position
17:2(A4,8)
17:2(4⁹.13)
18:2(A⁹.13)
O Position #2 is not linked to a fatty acid.
O 18:2(459)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 6 images

Knowledge Booster
Similar questions
- Why is the net charge 0 for cooh? Shouldn't it be +1?arrow_forwardIs B monomer a beta fructose or alpha fructose. How do you know?arrow_forwardModify isoleucine to show the predominant forms at pH 1, 7, and 13. Isoleucine has, values of 2.4 (carboxyl group) and 9.7 (amino group). Isoleucine at pH 7 Isoleucine at pH 1 CH3 CH₂ + H₂C CH +H₂N- C H Incorrect Isoleucine at pH 13 Incorrect H₂C H₂N- CH₂ | CH₂ CH c- C H 0 ĭ Incorrect H₂C +H₂N-arrow_forward
- What type of bond is created to form maltose? What is the waste product? * Maltose consists of two molecules of glucose that are linked by an a-(1,4') glycosidi (5 d.arrow_forwardShow anabolism of two molecules of serine. Be sure to clearly identify each molecule and bond/linkage involved. Hand-draw the diagramarrow_forwardConsider the positively charged amino acid lysine Lys2+ 21 COOH I H&N-C-H I pH 14 12 10 8 6 4 2 0 CH₂ I CH₂ I CH₂ I CH₂ T NH₂+ 0 Nelson p85 2.18 = 2.18 PK₁ Lys+ COO™ I H₂N-C-H H₂N-C-H ī I -----) 8.95 Lysº 8.95 pK₂ pka carboxyl = 2.19 pkaamino = 9.67 pka sidechain = 4.25 COO™ I CH₂ I CH₂ I CH₂ I CH₂ I NH₂¹ 1.0 2.0 Equivalents of OH added- COO™ I H₂N-C-H I 10.79 1 10.79 pk Isoelectric point Lys CH₂2 I CH₂ I CH₂ I CH₂ T NH₂ 3.0 +H3N NH3+ T CH₂ T CH₂ CH₂ CH₂ -COO™ H Lysine (Lys, K) Physiological pH = 7.4 < pl → Amino acid is positively charged at physiological pH 1. Consider glutamate in its fully protonated form (e.g. in a pH = 1 solution) 1) Draw all the forms of glutamate at various pH 2) Calculate the pl of this amino acid 3) Sketch a titration curve showing pH as a function of added [OH-] and locate the predominant forms of histidine in the curve STEPS: 1. Find the H atoms that can be removed on the molecule 2. Associated a pka value to each removable H. 3. Draw the Aa structure at:…arrow_forward
- 1arrow_forwardIdentify and encircle the peptide bonds in this polypeptide (Asp-Sec-Leu-Cys-Glu).arrow_forwardLefer to the figure showing the molecular structure of dimethylmercury. H–C-Hg C-H H. Exposure of human skin to a single drop of dimethylmercury can lead to death, as it is highly poisonous and passes easily cell membranes. Based on its structure, why is it able to pass so easily through cell membranes? I-0-I IICIHarrow_forward
- The φ angle represents rotation about which bonds? (mark these bonds with a φ directly on this structure)arrow_forwardDraw the oligopeptides' structure and provide the corresponding name for each oligopeptide 1. Dipeptide Ala-His 2. Tripeptide Glu-Pro-Cys Note: First residue is the N-terminal amino acidarrow_forwardIdentify the following amino acids and their following side chain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON