
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
predict the product of these reaction
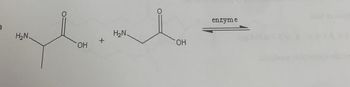
Transcribed Image Text:)
H₂N
OH
+
H₂N.
OH
enzyme
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- show how you would use bromination followed by amination to synthesize glycine show how you would use strecker synthesis to make phenylalanine and prepare a mechanism for each of the steparrow_forwardwith diagarmarrow_forwardAnother antidote for methanol poisoning is fomepizole, which is an aldehyde dehydrogenase (ADH) inhibitor. Would fomepizole be more or less effective than ethanol? Explain your reasoning.arrow_forward
- Metabolic pathways frequently contain reactions with positive standard free-energy values, yet the reactions still take place. How is this possible?arrow_forwardDifferentiate between homotropic and heterotropic and explain how they modify the equilibrium between the T and R forms of an allosteric enzyme.arrow_forwardWhich of the following is not an assumption made when evaluating Michaelis Menton kinetics? the reaction is happening at body temperature the substrate is in great excess of the catalyst measurement of initial velocity of the reaction reaction conditions are occurring under steady statearrow_forward
- Reactions taking place in dilute solution obey the rules ofsimple reaction kinetics because their rates depend on diffusion. How does this approach relate to investigations of enzymes in living cells?arrow_forwardExplain the details in the individual steps in the Michaelis-Menten mechanism of converting substrates into productsarrow_forwardExplain the krebs cycle. You must know the reactants, products and location for each step.arrow_forward
- Compile a list of all the cofactors involved in adding or removing one-carbon groups in carbohydrate, lipid, and amino acid metabolism. Provide an example of a reaction that uses each cofactor.arrow_forwardY Explain and show why phosphoenolpyruvateis a high-energy compound.arrow_forwardGive an example of one enzyme that participates in both glycolysis and gluconeogenesis. Evaluate the structures of chemical reactants and products and the enthalpic and entropic contributions to Gibbs free energy change for the reaction. Explain how the enzyme is able to catalyze the reaction (either direction.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON