
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Fill in the missing reactant, reagent, or product for the following reaction.
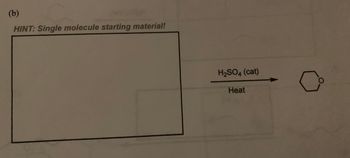
Transcribed Image Text:### Organic Chemistry Reaction Pathway
**Problem (b):**
**Hint:** Single molecule starting material!
**Reaction Details:**
- **Starting Material:** Not specified in the image.
- **Reagent/Catalyst:** H₂SO₄ (sulfuric acid), acts as a catalyst.
- **Condition:** Heat is applied.
- **Product:** The product is a six-membered cyclic ether, specifically tetrahydropyran.
**Diagram Explanation:**
- A reaction arrow points from the starting material (not shown) to the product, tetrahydropyran.
- Above the arrow, the catalyst “H₂SO₄ (cat)” is listed, indicating that sulfuric acid is used in catalytic amounts to facilitate the reaction.
- Below the arrow, the condition “Heat” suggests that the reaction requires elevated temperatures to proceed.
This setup suggests an intramolecular ring closure to form a cyclic ether from a linear precursor. The presence of sulfuric acid as a catalyst indicates an acid-catalyzed dehydration or substitution process.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What are 5 reactions that can create ethanoic acid? Draw them out pleasearrow_forwardDraw the product for the following reaction between an alkyne and one equivalent of HCI. HCI 3-methyl-1-pentynearrow_forwardSelect a name that is a correct alternative to 4-isopropylheptane. 2-methylpropylhexane 4-(methylethyl)heptane 2-methyl-3-propylhexane 3-propyl-2-methylhexane 5-methyl-4-propylhexanearrow_forward
- Write the reaction of an Alkyl halide ( any) with Ammonia.arrow_forwardWhat is the role of the catalyst in electrophilic aromatic substitution reactions of benzene? O a. The catalyst prevents addition reaction from occurring. b. The catalyst reacts with the non- benzene reactant to form the electrophile in the reaction. O c. The catalyst attaches to the benzene ring, making it more susceptible to electrophilic substitution O d. The catalyst speeds up the reaction by e deprotonating the arenium ion that forms in the reaction.arrow_forwardHow to Identify Electrophile & Nucleophile and know the differencearrow_forward
- The production of an ether from an alkyl halide. nucleophilic substitution reaction O radical substitution reaction O addition reaction O elimination reactionarrow_forwardSelect the bond(s) that is/are formed in the electrophilic addition. H :CI:arrow_forwardComplete the following syntheses – they may be two- or three-step processes. Include any necessary catalysts or reaction conditions. c) Prepare methyl ethanoate from ethanol and methanolarrow_forward
- 1. Complete the following reactions by writing missing reactant(s), reagent(s), or product(s).arrow_forwardAlkenes are inherently reactive because...? A) The electrons in n bonds are not as stable as in o bonds B) They make strong electrophiles C) The o bonds are unstable because their electrons are between nuclei D) They are always looking to react with nucleophilesarrow_forward10. Explain why aldehydes and ketones are electrophiles. In your discussion use resonance structures. H3C- CH3 H3C-arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY