
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
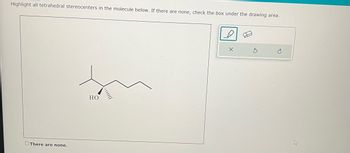
Transcribed Image Text:Highlight all tetrahedral stereocenters in the molecule below. If there are none, check the box under the drawing area.
There are none.
HO
3
4
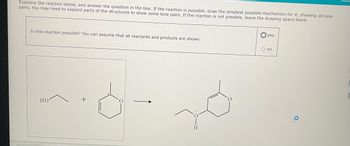
Transcribed Image Text:Examine the reaction below, and answer the question in the box. If the reaction is possible, draw the simplest possible mechanism for it, showing all lone
pairs. You may need to expand parts of the structures to show some lone pairs. If the reaction is not possible, leave the drawing space blank.
Is this reaction possible? You can assume that all reactants and products are shown.
HO
H
yes
O no
9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 15arrow_forwardmay you please answer these questionsarrow_forward1. In IR spectroscopy, the C=O bond has a stretching frequency than the C=S bond because A) higher, an O atom has less mass than an S atom B) lower, an O atom has more mass than an S atom C) higher, an O atom has less electronegativity than an S atom D) lower, an O atom has more electronegativity than an S atom E) higher, an O atom has an even number of neutronsarrow_forward
- Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Br t CI H Molecule 1 CI Explanation H Molecule 4 Hi OH Br CO " CI 'Br Onone of the above Check Q Search Molecule 2 H CI com Molecule 5 CI Br H Br Molecule 3 CI Br Br Molecule 6 ******* H H "CI X 3 © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilty BEEN alo Ar (arrow_forward(Please correct answer and don't use hend raiting) Can you please list all the chiral centres within each compound. :) OA. OC. OE. B. OD.arrow_forward1. How many chiral centers are there in the following molecule? Identify them with an asterisk. a) لم بشه Br b)arrow_forward
- Inspect the structures shown below and select the correct statement. COƏH HN-C-H CH2 ÇOH H2N-C-H CH2 COH HN-C-H CH2 COH HN-C-H Он SH 1 2 4 O #1 and #4 have the S configuration, #2 and #3 have the R configuration. O All structures have the R configuration. O All structures have the S configuration. O #1, #2, and #4 have the S configuration and #3 has the R configuration. O #1 and #4 have the R configuration, #2 and #3 have the S configuration.arrow_forwardWhich is the correct newman projection for the following molecule (as drawn below assuming no rotation) when looking down the bond indicated by the arrow? 0 0 Me Me Et Me Me Et Me Me II….. H H H Harrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY