
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Need to replace the two circled Cl's with Br at both places. The rest of the image reaction should stay as is. I attached the image without the circled Cl's in case that is needed to replace the two Cl's with Br.
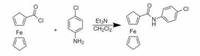
Transcribed Image Text:CI
Et3N
CH;Cl2
Fe
Fe
NH2
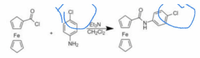
Transcribed Image Text:EGN
CH C2
Fe
Fe
NH2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the curved arrows and the resulting resonance structure of meta-nitrobenzoate that shows a carbocation that does not increase reactivity as much as para-nitrobenzoate. Include lone pairs in your answer. CH Edit Drawing Save for Later Attempts: 0 of 4 used Submit Answer MacBook Air 4)) F2 F10 & 8. delete } Y enter J L return M shift alt command option + ||arrow_forwardDraw the products of the reaction shown. Electron flow is indicated with curved arrows. HCI: . Include all valence lone pairs in your answer. Include counter-ions, e.g., Na+, I, in your submission, but draw them in their own separate sketcher. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the+sign from the drop-down menu. 0 + ? [ ChemDoodle Previous Nextarrow_forward3.- Draw all resonance forms for each species. For the anion and cation species, used curved arrows. For the radical species, use hooks. anion cation radicalarrow_forward
- Please help with all parts i am using this to studyarrow_forwardDraw the contributing structure that results from resonance indicated by the curved arrow(s). You do not have to consider stereochemistry. Explicitly draw all H atoms. You do not have to include lone pairs in your answer.arrow_forward1. Draw the best possible resonance contributor. Draw curved arrows to show electron movement.arrow_forward
- For this reaction: CaCl2 ( aq) + K3PO4( aq) --> What one of the following species would appear in the molecular equation? (Don't worry about coefficients. Just write the molecular equation, and see which of. these appears in it.) O Ca*( aq) O K2CI (s) O Ca3(PO4)2 (s) O KPO4(s) OK*(s)arrow_forwardCould we cut just one bond in the "starting" molecule shown in the drawing area below to create this "target" molecule? The target molecule. If so, highlight the bond to be cut. If not, check the box under the drawing area that says Not possible. Note: it's OK if cutting the bond creates more than one molecule, as long as one of them is the target molecule. Not possible. I Don't Know Submitarrow_forwardis this z or E?? If it is E. please in details explain why cycloproyl is greater than OH group???arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY