
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
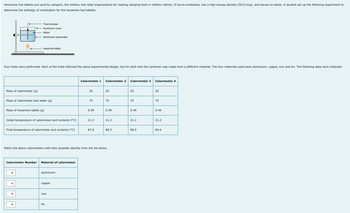
Transcribed Image Text:Hexamine fuel tablets are used by campers, the military and relief organizations for heating camping food or military rations. It burns smokeless, has a high energy density (30.0 kJ/g), and leaves no ashes. A student set up the following experiment to
determine the enthalpy of combustion for the hexamine fuel tablets.
Mass of calorimeter (g)
س
Four trials were performed. Each of the trials followed the same experimental design, but for each trial the container was made from a different material. The four materials used were aluminium, copper, iron and tin. The following data were collected.
Thermometer
Aluminium cover
Water
Aluminium calorimeter
Mass of calorimeter and water (g)
hexamine tablet
Mass of hexamine tablet (g)
Initial temperature of calorimeter and contents (°C)
Final temperature of calorimeter and contents (°C)
+
♦
Calorimeter Number Material of calorimeter
"
aluminium
copper
iron
Calorimeter 1 Calorimeter 2
tin
25
75
0.49
Match the above calorimeters with their possible identity from the list below.
21.2
87.8
25
75
0.49
21.2
88.3
Calorimeter 3
25
75
0.49
21.2
89.5
Calorimeter 4
25
75
0.49
21.2
84.6

Transcribed Image Text:561 J of energy was added to one of the four calorimeters from this experiment when it was empty and at an initial temperature of 21.2°C. The maximum temperature reached by the empty calorimeter was 70°C. From this data, the calorimeter is identified
as being composed of
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Similar questions
- A bomb calorimeter, or constant volume calorimeter, is a device often used to determine the heat of combustion of fuels and the energy content of foods. Since the "bomb" itself can absorb energy, a separate experiment is needed to determine the heat capacity of the calorimeter. This is known as calibrating the calorimeter. In the laboratory a student burns a 0.346-g sample of bisphenol A (C15H1602) in a bomb calorimeter containing 1090. g of water. The temperature increases from 24.60 °C to 26.80 °C. The heat capacity of water is 4.184 J gl°C!. The molar heat of combustion is –7821 kJ per mole of bisphenol A. C15H1602(8) + 18 02(g) →15 CO2(g) + 8 H2O(1) + Energy Calculate the heat capacity of the calorimeter. heat capacity of calorimeter = J/°Carrow_forward2. Fruit is impeded from ripening when it reaches high temperatures (>32°C). Farmer Joe is roo worried that Princeton's summer heat wave will affect the ripening of his ground cherries. The spherical fruit (1 cm in diameter) is surrounded by a spherical papery husk (husk = 0.3 W/m K) with an inner diameter of 2 cm and an outer diameter of 2.1 cm. The air in between the fruit and the husk is stagnant (air = 0.03 W/m K). On a calm day in July, the Princeton air (outside of the husk) is very warm, with a temperature of ~38°C and a heat transfer coefficient of hPrinceton,calm = 10 W/m².K. (a) Sketch the system, labeling all temperatures, dimensions, and proportionality constants.arrow_forwardWhat is the final temperature of a 200.00 gram sample of water (c = 4.18 J/g°C) that absorbs 5,000 Joules of heat energy after starting with a temperature of 20°C?arrow_forward
- A bomb calorimeter, or constant volume calorimeter, is a device often used to determine the heat of combustion of fuels and the energy content of foods.arrow_forwardin text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!!!!!!!arrow_forwardPlease help find Ccal. I was not sure what information is required so I have attached it below.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The