
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Consider a hypothetical
→+AB+CD
(In this equation
A, B, C and D
stand for some unknown chemical formulas.)
Here is an energy diagram for the reaction:
Use the energy diagram to answer these questions.
|
|||||||||||||||
|
|
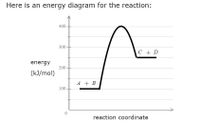
Transcribed Image Text:### Energy Diagram for a Chemical Reaction
#### Description
The diagram shown is an energy profile of a chemical reaction. On the y-axis, the energy is measured in kilojoules per mole (kJ/mol). The x-axis represents the reaction coordinate, which tracks the progress of the reaction from reactants to products.
#### Key Elements of the Diagram:
1. **Reactants**:
- The starting point on the diagram is labeled \( A + B \).
- At this point, the energy level is approximately 100 kJ/mol.
2. **Transition State**:
- The peak of the diagram represents the transition state, where the energy is the highest.
- The energy at this peak is around 400 kJ/mol.
3. **Products**:
- The ending point on the diagram is labeled \( C + D \).
- The energy level for the products is approximately 150 kJ/mol.
#### Explanation:
This energy diagram illustrates the energy change that occurs during the reaction from \( A + B \) to \( C + D \). Initially, the reactants \( A + B \) have an energy level of around 100 kJ/mol. As the reaction progresses, the system absorbs energy until it reaches the transition state with an energy of approximately 400 kJ/mol. After this peak, the energy decreases and stabilizes at 150 kJ/mol for the products \( C + D \).
This diagram shows that the reaction is **endothermic**, as the products have a higher energy (150 kJ/mol) compared to the reactants (100 kJ/mol).
Understanding such energy diagrams is crucial for grasping the concepts of activation energy and the energy profile of chemical reactions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Free Energy (kcal/mol) 25 20 15 10 5 0 B M A A Reaction progress Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, AG ‡, for the step B to A. kcal/mol Calculate the overall energy change, AGº, for the process A to D. Which step is faster, (a) B to C or (b) D to C? kcal/molarrow_forwardFree Energy (kcal/mol) 25 20 15 10 B 5 сле C A D 0 Reaction progress Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, AG *, for the step B to A. kcal/mol Calculate the overall energy change, AG°, for the process A to D. Which step is faster, (a) B to C or (b) D to C? @ kcal/molarrow_forwardConsider a hypothetical chemical reaction: A+B → C+D (In this equation A, B, C and D stand for some unknown chemical formulas.) Here is an energy diagram for the reaction: energy (kJ/mol) 400 300 200 100 0 C + D SCA A + B reaction coordinate Use the energy diagram to answer these questions. What is the heat of reaction? Is the reaction exothermic or endothermic? Can you determine the activation energy? Can you determine the activation energy of the reverse reaction? C+DA+B kJ/mol Exothermic Endothermic Neither Yes, No. it's No. kJ/mol Yes, it's kJ/molarrow_forward
- Which of the following statements is true about reaction rates? A Reaction rates can be predicted from the coefficients of the balanced equation. B Reactants usually disappear faster than the products appear. C Reactants usually disappear slower than the products appear. D Reaction rates can only be determined by experimentation.arrow_forward⦁ 4. Make your own energy diagram for an exothermic reaction that does not include a catalyst and one with a catalyst (Be sure each line is a different color or labeled). Label the axis of the diagram. Then, state what a catalyst is and how a catalyst affects the reaction rate.arrow_forwardMatch each label in diagrams 1 and 2 (a-i) with the appropriate description. Each answer choice is used only once OR not at all. ΔH > 0 activation energy product exothermic reaction activated complex 1. a 2. b 3. c 4. d 5. e 6. f 7. g 8. h 9. iarrow_forward
- 1. a. Explain in your own words, what is meant by the term "activation energy." b. How can the idea of activation energy be used to explain the temperature dependance of rate? c. How can activation energy be used to explain why a catalyst increases the rate of a chemical reactionarrow_forwardDetermine each type of reaction. NH,NO3 (s) → N,O (g) +2 H2O (1) Choose... CO (g) + 2 H2 (g) → CH3OH (1) Choose.. CaCl2 (aq) + Na,CO3 (aq) → 2 NaCl (aq) + CACO3 (s) Choose.. 2 C2H2 (g) + 5 02 (g) → 4 CO2 (g) + 2 H2O (1) Choose... | 2 Fe (s) + 6 HC1 (aq) → 2 FeCl3 (aq) + 3 H2 (g) Choose...arrow_forward19arrow_forward
- 400 300 + energy 200 - (kJ/mol) с +D 100 A + В reaction coordinate Use the energy diagram to answer these questions. I KJ/mol What is the heat of reaction? Exothermic Is the reaction exothermic or endothermic? Endothermic Neither Yes, it's kJ/mol Can you determine the activation energy? No. Can you determine the activation energy of the reverse reaction? Yes, it's kJ/mol С+D- A+B No.arrow_forward3. Draw a reaction energy diagram, and label the product (P), reactant (R), transition states (TS1, TS2, etc.), intermediates (INT1, INT2, etc.) and activation energies (AG‡₁, AG 2, etc.) for a reaction with the following criteria: i. A three-step, endothermic reaction; ii. AG3 > 0 but AG₁ AG‡₁ >> AG‡₂. Which species does the structure of the transition state of the rate-determining step resemble?arrow_forwardFor the reaction between aluminum metal and copper chloride (CuCl2) that we performed together, what is the reason that breaking a single 5 g piece of aluminum into 25 smaller pieces of aluminum (still with a total mass of 5 g ) speeds up the reaction rate.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY