
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Please draw the chemical mechanism for C
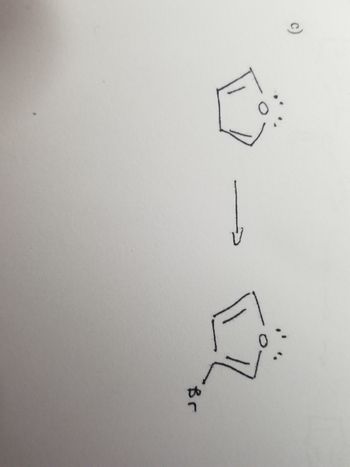
Transcribed Image Text:고
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Plz help with hw Calculate the energy (in kJ/mol) that electrons acquire as a result of being accelerate through a potential of 80 V. (b) How does this energy compare to that of a typical chemical bond?arrow_forwardEstimate ΔrH° for forming 2 mol ammonia from molecular nitrogen and molecular hydrogen. Is this reaction exothermic or endothermic?arrow_forwardFor the molecules HCN and CH3NH₂arrow_forward
- Use the bond energies provided to estimate DeltaH^ rxn for the reaction below. 2Br_(2(1))+C_(2)H_(2(g))rarrC_(2)H_(2)Br_(4(1))DeltaH^rxxn=?arrow_forward4.33 The absolute configuration of (-)-bromochlorofluoromethane is R. Which of the following is (are) (-)- BRCIFCH? Br F F Br F H C1FH С—СІ F .... CI Il.. Br H H Brarrow_forwardThe bond dissociation energy to break 1 C-O bond(s) in 1 mole of glycerol (HOCH₂CH(OH)CH₂OH, see image below) molecules isarrow_forward
- Ansider he realhon N2 cg) +3 H2 (9)-)2NH, C9) If H.05 of N, realt lolth ese cess Hz, How many pames Of NHz will form.arrow_forwardWhat is the molecular and electronic configuration for H2COarrow_forwardThe Lewis structure for the chlorate ion is Calculate the formal charge on the chlorine (C1) atom. Express your answer as an integer. ► View Available Hint(s) formal charge on Cl = Submit Previous Answers Incorrect; Try Again; 3 attempts remaining Part B Calculate the formal charge on each of the oxygen (O) atoms labeled a, b, and c in the following Lewis structure. b :O: Express your answers as integers separated by commas. ► View Available Hint(s) b :0: formal charge on Oa, Ob, Oc c =arrow_forward
- The energies of the MOs of O2 are indicated in the table in Annex. What is the energyrequired to form one O2+ ion from one molecule of O2? Give your answer in J.arrow_forwardUse the molar bond enthalpy data in the table to estımate the value of AHn for the rxn equation CH (g) + 4 Cl, (g) → CCI, (g) + 4 HCl(g)arrow_forwardAre the 5 hydrogen atoms of C2H5Cl equivalent?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning