
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Hellllllppppppp
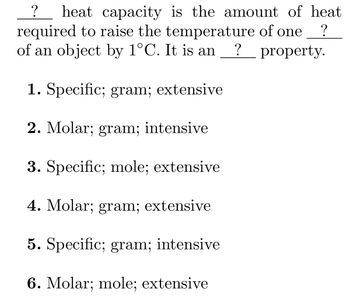
Transcribed Image Text:?
heat capacity is the amount of heat
required to raise the temperature of one ?
of an object by 1°C. It is an ? property.
1. Specific; gram; extensive
2. Molar; gram; intensive
3. Specific; mole; extensive
4. Molar; gram; extensive
5. Specific; gram; intensive
6. Molar; mole; extensive
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Let's assume Cu(OH)2 is completely insoluble, which signifies that the precipitation reaction with NaOH presented in the transition would go to completion Cu2+ +2NaOH Cu(OH)2 + 2Na+ if you had 0.450L solution containing 0.0240 M of Cu2+ and you wished to add enough 1.32 M NaOH to precipitate all of the metal, what is the minimum amount of the NaOH solution you need to add? Assume that the NaOH solution is the only source of OH- for the precipitation express the volume to three significant figures and include the appropriate unitsarrow_forwardWhat is the solubility product expression for Co(OH)3? Ksp= [Co3+][30H] Ksp= 3[Co3+][OH73 Ksp= [Co3+][OH73 Ksp= [Co3+][30H]3 Ksp= [Co3*][OH]arrow_forwardUse the following data to calculate the Ksp value for each solid. -3 a. The solubility of MgC2O4 is 9.3 × 10-³ mol/L. b. The solubility of Au(OH)3 is 2.12 × 10-¹² mol/L. K sp K sp - =arrow_forward
- For the aqueous [HgI4]complex Ky = 6.76 × 1029 at 25 °C. Suppose equal volumes of 0.0062 M Hg (NO3)2 solution and 0.42M Nal solution are mixed. Calculate the equilibrium molarity of aqueous Hg** ion. Round your answer to 2 significant digits. ☐ Marrow_forwardWhat is the fluoride ion concentration for a saturated solution of SrF2 if the Ksp for SrF2 is 2.5 x 10-9? a. 6.3 × 10-26 M b. 1.7 × 10-3 M c. 8.5 × 10-4 M d. 3.4 × 10-4 Marrow_forwardmagnesium hydroxide, Mg(OH)2, found in milk of magnesia, has a solubility of 7.05x10-3 gL-1 at 25ºC. Calculate the Ksp for Mg(OH)2 with a tolerance of +8 (Please type answer)arrow_forward
- Which of the following is the correct Ksp expression for the insoluble solid, Ag,SO,? A) K sp 2[Ag*][SO}¯] B) K sp = [Ag*][SO}¯] C) Ksp = [Ag*]*[SO?¯] [Ag*]*[SO?¯] D) K en = Ag,SO4 ds 2[Ag*][S0}¯] K sp E) Ag,sO4 A) see problem image A) B) see problem image B) C) see problem image C) D) see problem image D) E) see problem image E)arrow_forwardBarium sulphate is a relatively insoluble salt used for medical radiographs of the gastrointestinal tract: BaSO4 (s) Ba2+ (aq) + SO4²- (aq) The concentration of Ba2+ ions in a saturated aqueous solution is 1.05×105 M. Determine Ksp for barium sulphate dissolving in water. O 1.12 x 10-5 O 1.05 x 1025 O 2.12 x 10-5 1.10 x 10-25 O 1.10 x 10-10arrow_forwardWhich of the following is the correct solubility product constant expression for PBSO4? O Kp = [Pb2]°[So,212 %3D Kp = [Pb2*][SO,2 ] O Ksp = 2[Pb2'12[SO,2] %3D Ksp = 2[Pb2']² 2[So,212 %3Darrow_forward
- Please don't provide handwritten solution ....arrow_forwardLl.120.arrow_forwardWithout doing any calculations (just compare the correct Ksp values) complete the following statements: 1. magnesium fluoride is MORE soluble than A 2. magnesium fluoride is LESS soluble than B A. PbBr2 B. Ni(CN)2 C. COCO3 D. COSarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY