
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
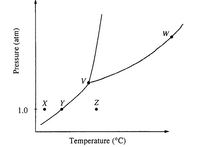
The phase diagram for a pure substance is shown above. Use this diagram and your knowledge about changes of phase to answer the following questions.
a) What does point V represent? What characteristics are specific to the system only at point V?.
(b) What does each point on the curve between V and W represent?
(c) Describe the changes that the system undergoes as the temperature slowly increases from X to Y to Z at 1.0 atmosphere.
Note: Please briefly explain A-C. Thank you.
Transcribed Image Text:W.
V
X
Y
1.0
Temperature (°C)
Pressure (atm)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- kJ The enthalpy of vaporization of Substance X is 5.00 and its normal boiling point is -66. °C. Calculate the vapor mol pressure of X at -87. °C. Round your answer to 2 significant digits. || atm x10arrow_forwardFour liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain th order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL. solution freezing point boiling point 3.6 g of calcium chloride (CaCl2) dissolved in 300. mL of water (choose one)v (choose one) v (choose one) (choose one) 3.6 g of propylene glycol (C3H8O2) dissolved in 300. mL of water 3.6 g of glucose (C6H1206) dissolved in 300. mL of water 300. mL of pure water (choose one) 4(highest) く (choose one) 1(lowest) x 5arrow_forwardFour liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL. solution 2.2 g of ethylene glycol (C₂H602) dissolved in 200. mL of water 2.2 g of sucrose (C12H22011) dissolved in 200. mL of water 2.2 g of potassium nitrate (KNO3) dissolved in 200. mL of water 200. mL of pure water freezing point (choose one) (choose one) (choose one) (choose one) X boiling point (choose one) (choose one) O (choose one) (choose one)arrow_forward
- There are three sets of sketches below, showing the same pure molecular compound (water, molecular formula H₂O) at three different temperatures. The sketches are drawn as if a sample of water were under a microscope so powerful that individual atoms could be seen. Only one sketch in each set is correct. Use the slider to choose the correct sketch in each set. You may need the following information: melting point of H₂O: 0.0 °C boiling point of H₂O: 100.0 °C A (Choose one) (Choose one) 188. °C B 5 35. °C 4 5 (Choose one) -25. °℃arrow_forwardEthanol, with ΔHvap = 40.5 kJ mol-1, has a higher ΔHvap than methanol, which is 35.2 kJ mol-1, due to its larger size and greater intermolecular forces. Because the ΔHvap for ethanol is larger, we would expect that for the two liquids to reach the same vapor pressure, the temperature for ethanol would have to be higher. If the vapor pressure is 0.0992 atm at 27.3 °C, what would the temperature be when the vapor pressure is 4.52 atm?arrow_forwardEnter your answer in the provided box. The vapor pressure of a liquid doubles when the temperature is raised from 75°C to 85°C. At what temperature will the vapor pressure be five times the value at 75°C?arrow_forward
- What is Δq for the vaporization of 50.0 g of Compound A (76.03 g/mol; ΔHvap is 31.6 kJ/mol)?arrow_forwardIn addition to filling in the blanks below, show all of your work for this problem on paper for later upload. The heat of vaporization of isopropanol is 44.0 kJ/mol. The vapor pressure of isopropanol at 400 torr is 67.8 °C. Calculate the normal boiling point of isopropanol. Enter your value in the first box and an appropriate unit of measure in the second box.arrow_forwardFour liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL. solution freezing point boiling point 6.4 g of glucose (C6H1206) dissolved in 300. mL of water (choose one) O (choose one) 6.4 g of potassium sulfate (K2SO4) dissolved in 300. mL of water (choose one) (choose one) O 6.4 g of sodium bromide (NaBr) dissolved in 300. mL of water (choose one) (choose one) O 300. mL of pure water (choose one) E (choose one) Earrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY