
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Add curved arrows to show this step in the mechanism
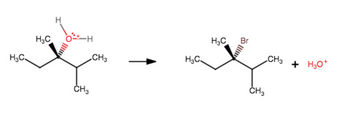
Transcribed Image Text:HC,
НС
Н
0 -н
CH3
CH3
HC,
НС
Br
CH3
CH3
+ H30+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Similar questions
- Complete the mechanism for this reaction by adding curved arrows and products. Add steps as necessary, and be sure to include lone pairs and charges where relevant. Ö-H of H-Cl Add/Remove step X Click and drag to start drawing a structure.arrow_forwardThe first and third boxes are correct. The second(middle box) step is incorrect. Can someone help me figure out the middle box.arrow_forward. One of these reactions occurs rapidly while the other is so slow that substitution products are not observed. Determine which reaction is which and explain the difference in rate using structural drawing and a few words. Br + 'Br +arrow_forward
- Show the mechanism for the given reaction conducted at -5 °C in CCI,. cyclohexene + bromine → dibromocyclohexane Draw structures, including charges and electrons, and add curved arrows. Details count. Draw each species (organic and inorganic) resulting from the previous step. Then, add curved arrows for the forward reaction. Include charges and nonbonding electrons. Add curved arrows to the first step. : Br - Br : Draw the major product. Include charges and nonbonding electrons.arrow_forwardTyped solutionarrow_forwardDraw the complete, detailed mechanism (curved arrows) for the following reaction. Br 2. H₂O OKarrow_forward
- The substitution reaction studied here with Chlorobutane and KOH is known to have a second order rate equation meaning the transition state in the slow step involves both nucleophile and electrophile. Knowing that, which of the following statements are true? Select all that apply. A) Adding more Chlorobutane (the electrophile) will not change the rate of the reaction. B Adding more KOH (the nucleophile) will make the reaction go faster. Adding more Chlorobutane (the electrophile) will make the reaction go faster. D) Adding more KOH (the nucleophile) will not change the rate of the reaction. E Adding more KOH (the nucleophile) will make the reaction go slower.arrow_forwardSelect any and all of these compounds that can undergo an addition and elimination reaction mechanism. OH OCH, A B C D Earrow_forwardShow the mechanism for this reaction of alkynesarrow_forward
- Label each one as either ortho, meta, or para.arrow_forwardWrite the mechanism for the following reaction step using curvedarrows, and draw the result of those motions. You will need to draw adifferent conformation of the reactant.arrow_forwardConsider the mechanism of the reaction shown below. Give the structure of the next important organic reaction intermediate along the reaction coordinate. Your answer could be the final produ HBr Edit Click on the drawing box above to activate the MarvinSketch drawing tool and then draw your answer to this question. If there is no reaction, then check the "no reaction" box below. no reactionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY