
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
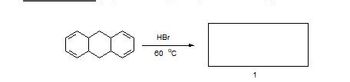
Transcribed Image Text:HBr
60 °C
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Convert 475 cal to joulesarrow_forwardhow do you convert Joules to reciprocal cm (cm-1) ?arrow_forwardYou go climbing in the Andes, and stop to make tea. You find that the water boils at a temperature of 83.9 °C. What is the atmospheric pressure where you are? (Given: ΔH°vap(H2O) = 43.4 kJ/mol) (2 decimal places)arrow_forward
- 2 ने \S h CoefRdlen+ 02 A०० e what is balanding the coefficient Agel tHhe follawing equuation? के AgNO,Ca AgClarrow_forwardWhat is the final temperature (in °C) of 590.1 g of water (specific heat = 4.184 J/g・°C) at 24.20°C that absorbed 950.0 J of heat?arrow_forward2,4-Dichlorophenoxyacetic acid ("2,4-D") is a widely used herbicide with the molecular formula C8H6Cl2O3C8H6Cl2O3. The pKapKa of 2,4-D is 2.73. A certain water-based herbicide product contains 130 gL−1gL−1 of 2,4-D. What is the pHpH of the 2,4-D solution?arrow_forward
- What is the boiling point of the unknown substance Xin K(Kelvin)? X(e) = X(g) AH;°(kJ mol¯1) S°(J mol-1 K-1) X(g) -82.0 238 X(e) -101 144arrow_forwardHow many joules of heat are required to change 563 grams of solid ice with a temperature of260 Kelvin into that many grams of liquid water with a temperature of 300 Kelvin? [For thisquestion, you may use 273 Kelvin as the melting point of ice.]arrow_forwardConsider the graph below. Note that the x-axis units are reciprocal kelvins. The equation of the linear fit is y = 5.43 x 10“ z + 567. 1/T Calculate the value of A, H° in kJ/mol. In K bəarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY