
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Dear expert pro hand written solution is not allowed.
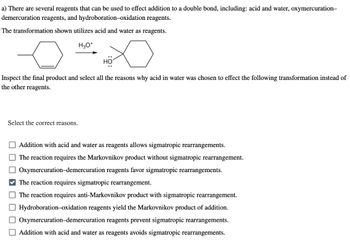
Transcribed Image Text:a) There are several reagents that can be used to effect addition to a double bond, including: acid and water, oxymercuration-
demercuration reagents, and hydroboration-oxidation reagents.
The transformation shown utilizes acid and water as reagents.
H3O+
HO
Inspect the final product and select all the reasons why acid in water was chosen to effect the following transformation instead of
the other reagents.
Select the correct reasons.
Addition with acid and water as reagents allows sigmatropic rearrangements.
The reaction requires the Markovnikov product without sigmatropic rearrangement.
Oxymercuration-demercuration reagents favor sigmatropic rearrangements.
The reaction requires sigmatropic rearrangement.
The reaction requires anti-Markovnikov product with sigmatropic rearrangement.
☐ Hydroboration-oxidation reagents yield the Markovnikov product of addition.
Oxymercuration-demercuration reagents prevent sigmatropic rearrangements.
Addition with acid and water as reagents avoids sigmatropic rearrangements.
UUU
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Following is a balanced equation for the allylic bromination of propene. CH2==CHCH3 + Br2 h CH2==CHCH2Br + HBr (a) Calculate the heat of reaction, H 0, for this conversion. (b) Propose a pair of chain propagation steps and show that they add up to the observed stoichiometry. (c) Calculate the H 0 for each chain propagation step and show that they add up to the observed H 0 for the overall reaction.arrow_forwardSubstitution Reactions Predicting the product of a nucleophilic substitution reaction Draw the organic product you would expect to isolate from the nucleophilic substitution reaction between the molecules shown. Note: You do not need to draw any of the side products of the reaction, only the substitution product. HO + + Br xx X S C Click and drag to start drawing a structure.arrow_forwardDraw the products of the three step reaction sequence shown below. Ignore inorganic byproducts. If the reaction results in a mixture of ortho and para isomers, draw only the para-product.arrow_forward
- The synthesis above used bromoethane, but acetylene is the only allowed source of carbon atoms. Using the reagents given, identify a synthetic route for the production of bromoethane from acetylene. The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagent(s) in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer.arrow_forwardConvert 2-pentanol into 2,3-dibromopentane. Draw structures of the starting material (2-pentanol) and final product (2,3-dibromopentane), and show the two reactions needed for this synthesis. Include the structure of the intermediate compound, and the reagents and conditions for each reaction. Then explain why 1,2-dibromopentane would not be a significant product of this synthesis.arrow_forwardCan you assist with the steps?arrow_forward
- Choose 2 of the 3 compounds and provide a synthesis starting with benzene and any other carbon compound with four or less carbons.arrow_forwardThe mechanism proceeds through a first cationic intermediate, intermediate 1. Nucleophilic attack leads to intermediate 2, which goes on to form the final product. In cases that involve negatively charged nucleophile, the attack of the nucleophile leads directly to the product. H. Br + CH3OH Br Intermediate 2 (product) Intermediate 1 In a similar fashion, draw intermediate 1 and intermediate 2 (final product) for the following reaction. OCH3 Cl2 MEOH ĆI racemic mixture Pay attention to the reactants, they may differ from the examples. In some reactions, one part of the molecule acts as the nucleophile. • You do not have to consider stereochemistry. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate intermediate 1 and intermediate 2 using the the dropdown menu. → symbol fromarrow_forward1a). Plan a synthesis of 3,4-dibromoaniline, starting from benzene. 4steps are required, lists reagents needed and draw in the intermediates.arrow_forward
- Draw the products of the three step reaction sequence shown below. Ignore inorganic byproducts. If the reaction results in a mixture of ortho and para isomers, draw only the para-product.arrow_forwardIn both examples below the reactants shown are combined to bring about a nucleophilic substitution (S1, Sy2) and'or elimination (EI, E2) reaction What is the major reaction that takes place in each case? CH, CH, CH,CH,CHCCH, H20 Br NaNg CH,OH CH,CH,CHCH,CH, SN2arrow_forwardProvide the major product for the reaction of the following starting material with excess HBr. If more than one product is formed, list the major product first. If no reaction, draw the starting material.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning